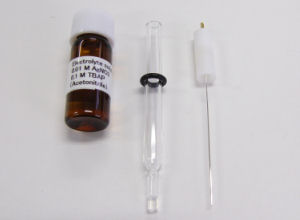
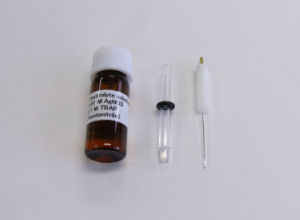
RE-7 Non Aqueous reference electrode / RE-7S Non Aqueous reference electrode
We developed new Non-Aqueous Reference electrode RE-7 and RE-7S as successors of our former
Non-Aqueous Reference electrode RE-5 and RE-5S.
In order to overcome fear of inner electrolyte's evaporation during their storage or transportation,
we separated them into the body and the inner electrolyte. This revolutionary modification
enables users to refill the soution and to use it repeatedly.
Spare products
Preservation of the electrode
· Fill the sample holder with an acetonitrile/0.1 M tetrabutylammonium perchlorate/0.01 M AgNO3 solution.
· Rinse the Ag wire with acetone.
· Keep the electrode immersed in an acetonitrile/0.1 M tetrabutylammonium perchlorate solution, with or without Ag ion (0.01 M AgNO3).
Note:
- Avoid the dry of the Vycor® glass, it could cause the crash of the Vycor® glass tip, caused by the salt crystallization inside of the glass. Make sure to keep the tip always wet.
- Before assembly the electrode, immerse and keep the Vycor® glass tip in an organic solvent for at least one hour.
- If bubbles are present around the Vycor® glass, remove with a slight tapping. Air bubbles will isolate, blocking the current flow.
- Use only high grade products and reagents.
- Wash with acetonitrile. Salt crystallization, in the inner side of the Teflon cap and at the edge of the sample holder, makes difficult the assembly.
Precautions with:
[Electrode]
This electrode is silver-silver ion reference electrode for non-aqueous solution.
Order to reduce the liquid junction potential, use the same solvent and supporting electrolyte, as a sample and electrode internal solution.
[Solvent]
The solvent in non-aqueous electrochemical should be considered as following:
1. Electrode activity material and produced substance solubility.
2. Select according to the potential area to be measured.
For example, dimethyl sulfoxide (DMSO) is major solvent in the negative potential area,
and acetonitrile (ACN) is major solvent in the positive potential area.
The non-aqueous solution could contains impurities, and the impurities could cause the block
reactions. Distilled the solvent sufficiently, to eliminated impurities before using.
[Supporting electrolyte]
The supporting electrolyte in the non-aqueous solvent system is required to meet the following requirements.
1. High solubility to the organic solvent
2. Wide potential window
3. Do not react with the organic solvent
Typical supporting electrolyte:
· Tetramethylammonium perchlorate (TEAP)
· Tetrabutylammonium perchlorate (TBAP)