Reference electrode for Non Aqueous solution
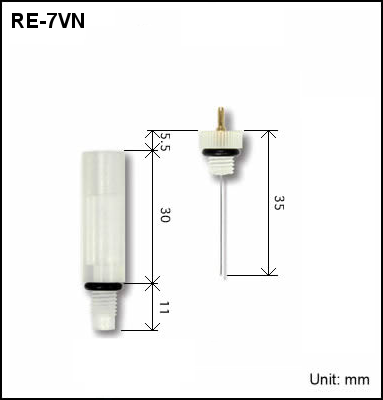
∗RE-7VN Non Aqueous reference electrode screw type was designed to be used in ALS brand flow cell (Cross, Radial, EQCMT, SEC-3F), for this reason there is a possibility that it will not be compatible in another brand flow cell.
- Feature
- Precautions
- Line up
- Assembly
- Internal solution
- For apply in an organic solvent sample
- Internal solution changeable
Preservation of the electrode
- Fill the sample holder with an acetonitrile/0.1 M tetrabutylammonium perchlorate/0.01 M AgNO3 solution.
- Rinse the Ag wire with acetone.
- Keep the electrode immersed in an acetonitrile/0.1 M tetrabutylammonium perchlorate solution, with or without Ag ion (0.01 M AgNO3).
Note:
- Avoid the dry of the IPPG (Ion Permeability Porous Glass); glass, it could cause the crash of the IPPG; glass tip, caused by the salt crystallization inside of the glass. Make sure to keep the tip always wet.
- Before assembly the electrode, immerse and keep the IPPG; glass tip in an organic solvent for at least one hour.
- If bubbles are present around the IPPG; glass, remove with a slight tapping. Air bubbles will isolate, blocking the current flow.
- Use only high grade products and reagents.
- Wash with acetonitrile. Salt crystallization, in the inner side of the Teflon cap and at the edge of the sample holder, makes difficult the assembly.
Electrode
This electrode is silver-silver ion reference electrode for non-aqueous solution.
In order to reduce the liquid junction potential, use the same solvent and supporting electrolyte, as a sample and electrode internal solution.
Solvent
The solvent in non-aqueous electrochemical should be considered as following:
- Electrode activity material and produced substance solubility.
- Select according to the potential area to be measured.
For example, dimethyl sulfoxide (DMSO) is major solvent in the negative potential area, and acetonitrile (ACN) is major solvent in the positive potential area.
The non-aqueous solution could contains impurities, and the impurities could cause the block reactions. Distilled the solvent sufficiently, to eliminated impurities before using.
Supporting electrolyte
If the sample is dissolved in an organic solvent, the supporting electrolyte must be added.
In order to select the supporting electrolyte, it is necessary to consider the following:
- Solubility in organic solvents
- Wide potential window
- No reaction with organic solvent
Typical supporting electrolyte:
- TEAP: Tetraethylammonium perchlorate
- TBAPF6: Tetrabutylammonium hexafluorophosphate
| Catalog No. | Description | Junction | Purpose |
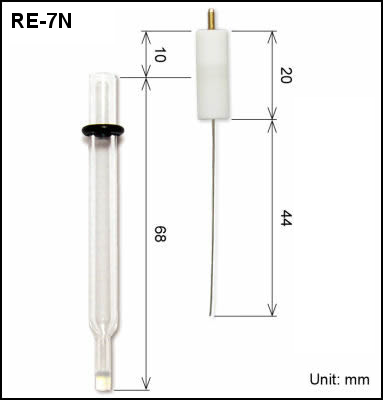
| 013848 | RE-7N Non Aqueous reference electrode | IPPG | SVC-2, SVC-3, VC-4, SBC Bulk electrolysis, RRDE, QCMT |
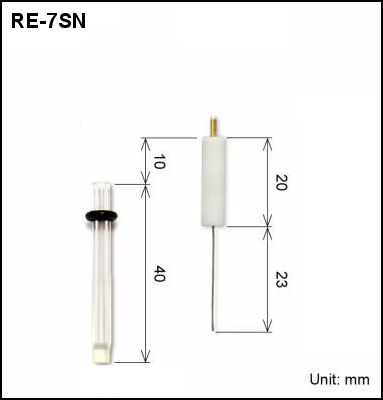
| 013849 | RE-7SN Non Aqueous reference electrode | IPPG | SECM |
| 013850 | RE-7VN Non Aqueous reference electrode screw type | Ceramics | Flow cell (Cross, Radial, EQCMT, SEC-3F) |
| Optional items (sold separately) | |||
| 012108 | RE-PV Preservative vial for reference electrode | For preservation of OD 6.0 mm reference electrode | |
| 012057 | RE-7 Teflon cap with Ag wire | With Ag wire | |
| 012058 | RE-7S Teflon cap with Ag wire | With Ag wire | |
| 012176 | Sample holder dia 6 mm (2 pcs) | IPPG | For double junction, manufacturing of the electrode |
| 012177 | Sample holder dia 9 mm (2 pcs) | IPPG | For double junction, manufacturing of the electrode |
- Contents for, Non Aqueous reference electrode
- Teflon cap with Ag wire (1 pc)
- Sample holder dia 6mm (1 pc)
- The internal solution is not included, please prepare the internal solution first. In the section Internal solution, there is an instruction for the preparation of an acetonitrile (ACN) solution containing 0.01 M silver(I) nitrate (AgNO3) and 0.1 M tetrabutylammonium perchlorate (TBAP), it can be used as a reference.
- Immerse the sample holder tip (IIPG) in an organic solvent to be used,at least for one hour.
- Fill the sample holder with 0.6 mL of the electrolyte solution, use the pipette and fill it carefully.
- If there is a presence of the air bubble on the extremity (liquid junction), flick the sample holder with the fingers.
- Set the Teflon cap and seal the cap with a parafilm, to avoid the evaporation of the inner solution.
(※If the reagents will be prepared by yourself, choose a special grade reagents.)
Attention for the re-assembly
- There is a possibility to have the precipitation of the support salt inside of the sample holder and Teflon cap, please wash with acetonitrile before re-use.
- If the extremity tip (liquid junction IPPG) is dried, suport salt deposition will happen, blocking the holes which increases the liquid junction resistance or breaks the tip (IPPG). Keep it moistening in a solvent.
(∗Ion Permeability Porous Glass clogging can not be recovered.)
Internal solution preparation
The internal solution of an Ag/Ag+ reference electrode used in a non aqueous solvent system consists mainly of a salt containing Ag+ ions and a supporting electrolyte added to the same non aqueous solvent as the solution sample to be measured.
Here, as an example, an acetonitrile (ACN) solution containing 0.01 mol/L silver(I) nitrate (AgNO3) and 0.1 mol/L tetrabutylammonium perchlorate (TBAP) is prepared.
NOTE:
- The reagents used for preparation are not available at our company. Please prepare commercially available special grade reagents separately.
- Please use acetonitrile that has been dried and dehydrated in advance.
- Be sure to wear protective equipment while working, and be careful when handling chemical substances.
- The amount of solution to be prepared is only a guide, so please consider it according to your experimental plan.
Preparation procedure


Fig. 1 What you need to prepare:
(1) Volumetric flask (25 mL, 50 mL)
(2) Spatula
(3) Medicine wrapping paper
(4) Beaker 50 mL (2 pcs)
(5) Pipette 1 mL;
(6) Glass rod (stirring rod);
(7) Light shielding bottle


Fig. 2 Weigh 1.71 g tetrabutylammonium perchlorate (TBAP) using an analytical digital balance and medicine wrapping paper. The TBAP is then transferred from the medicine wrapping paper to a 50 mL beaker, and a small amount of acetonitrile (ACN) solution is added to the beaker to dissolve the TBAP.


Fig. 3 Transfer the solution from the beaker to a 50 mL volumetric flask using a glass rod or similar to prevent spillage. Using a 1 mL pipette, adjust the volume of the volumetric flask to 50 mL while adding ACN solution to prepare a 0.1 mol/L TBAP/ACN solution.


Fig. 4 Weigh 0.043 g silver(I) nitrate (AgNO3) using an analytical digital balance and medicine wrapping paper. After weighing, transfer AgNO3 from the medicine wrapping paper to another 50 mL beaker and add a small amount of the 0.1 mol/L TBAP/ACN solution prepared earlier to dissolve the AgNO3.


Fig. 5 Transfer the solution from the beaker to a 25 mL volumetric flask using a glass rod or similar through the solution. Rinse the beaker further with acetonitrile (ACN) and repeat the process of transferring to a volumetric flask several times. Then, using a 1 mL pipette, add 0.1 mol/L TBAP/ACN solution while adjusting the volume of the volumetric flask to 25 mL.


Fig. 6 Transfer the 0.01 mol/L AgNO3/0.1 mol/L TBAP/ACN solution prepared in the previous section from a 25 mL volumetric flask to a light-shielded glass bottle for storage. The approximate storage time is about 1 month in a cool, dark place. Attach a label stating the composition and date of preparation or a toxicology-related label to the light-shielded glass bottle before storing.

