Part 3: Cyclic voltammetry - III
M.Sc. Takayuki Tezuka
4. Peak Current
The faradaic current at the peak value is referred to as the peak current, and it is known that the following equation can be adapted to the peak current of reversible reactions with fast electrons transfer. (Randles - Sevcik equation).
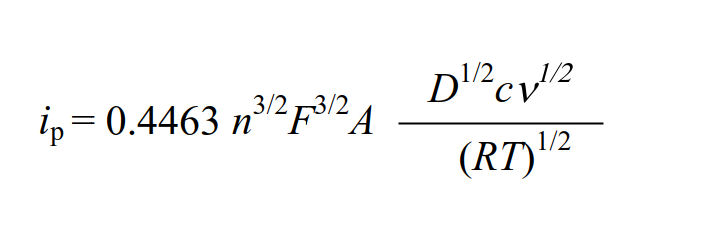
where: ip is peak current, F is Faraday constant (96485 C/mol),
A is working electrode surface area, D is diffusion coefficient,
c is concentration, v is scan rate, R gas constant (8.31 J.K-1.mol-1),
and T is temperature.
At 25°C, this equation summarizes the constants and is expressed as follows.
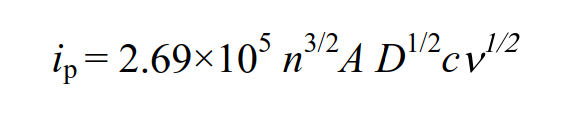
The peak current is proportional to the concentration c of the redox substance and the square root of the scan rate v.
Fig. 3-4-1 shows the result of cyclic voltammetry of 2 mmol/L potassium ferricyanide solution at scan rates of 0.01, 0.04, and 0.09 V/s. Fig. 3-4-2 plots the relationship between the square root of the scan rate and the reduction peak current, and it can be seen that there is a linear relationship. Also parameters such as the diffusion rate D can be get by drawing an approximate line for this plot and using its slope.
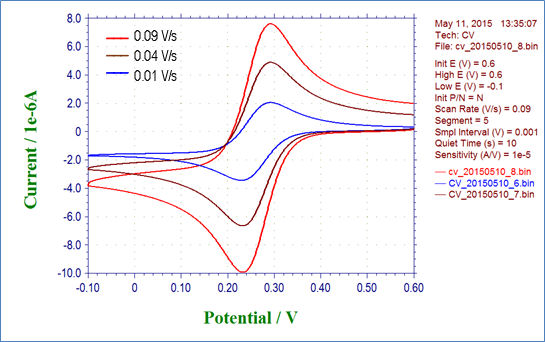
Fig. 3-4-1 Cyclic voltammogram of 2 mmol/L K3Fe(CN)6 varying the scan rate.
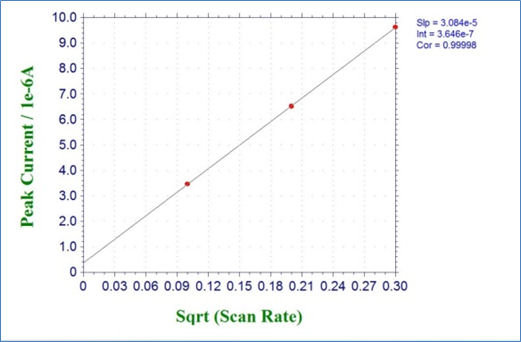
Fig. 3-4-2 Plots of reduction peak current versus square root of the scan rate.

