Sample preparation
Solutions to be used, in the Fundamental Electrochemical and Spectroelectrochemical measurement, will be prepared and adjusted in this section.
For the electrochemical, spectrochemical and spectroelectrochemical measurement the 2 mM Potassium ferricyanide (K3[Fe(CN)6]) in 1 M Potassium nitrate (KNO3) and 2 mM Potassium ferrocyanide (K4[Fe(CN)6]) in 1 M Potassium nitrate (KNO3) will be used.
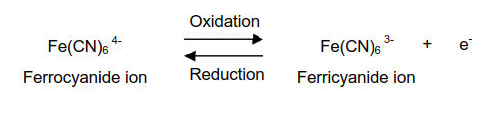
The oxidation of ferrocyanide ion to ferricyanide ion is a fast reversible electron transfer reaction at most electrodes. A typical cyclic voltammetry example is the current - potential curve of a solution containing ferrocyanide ion (2 mM potassium ferrocyanide in 1 M potassium nitrate). In this one-electron redox reaction, the ferrocyanide ion Fe(CN)64− is a reductant and the ferricyanide ion Fe(CN)63− is an oxidant.
1 M Potassium nitrate (KNO3)
Potassium nitrate (KNO3), electrochemically inert, is using as a supporting electrolyte, which is required to prevent transport of electroactive species by ion migrating in the electric field gradient.
1 M Potassium nitrate (KNO3) solution will be used to dissolve the salts of Potassium ferrocyanide (K3[Fe(CN)6]) and Potassium ferrocyanide (K4[Fe(CN)6]).


Fig. 1 What you need to prepare:
(1) Potassium nitrate
(2) Weighing paper
(3) Spatula
(4) Beaker 500 mL
(5) Metric cylinder 100 mL
(6) Distilled water


Fig. 2 Weigh the potassium nitrate (1) using folded and creased weighing paper (2) with an electronic balance.


Fig. 3 Transfer the potassium nitrate in to the Beaker 500 mL (4), and dissolve with 250 mL of distilled water (6). Measure the volume of distilled water (6) using a metric cylinder (5).
- Measure the 25.28 g (Fig. 2) of the potassium nitrate (1) using the weighing paper (2). Before set the weight paper (2) in an electronic balance, fold and crease the weighing paper (2). It will prevent that when you add the potassium nitrate (1) using a spatula (3), it spreads on the tray.
- Transfer the measured potassium nitrate to the beaker 500 mL (4) and add the distilled water (6) with a metric cylinder (5) in steps, until complete 250 mL. Using the weighing paper (2) will make easy to transfer the potassium nitrate (1) in to the beaker 500 mL (4) without spreading. Also, using the metric cylinder (5) which has a spout, it will prevent the spilling of the distilled water.
- The solution 1 M Potassium nitrate (Fig. 3) is ready to be used for the preparation of 2 mM Potassium ferrocyanide (K4[Fe(CN)6]) and 2 mM Potassium ferrycyanide (K3[Fe(CN)6]), both in 1 M Potassium nitrate (KNO3).
2 mM K3[Fe(CN)6] and 2 mM K4[Fe(CN)6] in 1 M KNO3
Potassium nitrate will be used as a supporting electrolyte, and 1 M Potassium nitrate solution will be used to dissolve the salts of Potassium ferrocyanide (K4[Fe(CN)6]) and Potassium ferricyanide (K3[Fe(CN)6]).


Fig. 4-1 What you need to prepare 2 mM Potassium ferricyanide:
(1) Potassium ferricyanide
(2) Spatula
(3) Beaker 50 mL
(4) Metric cylinder 100 mL
(5) Glass rod (stirring rod)
(6) Volumetric flask
(7) Pipette 5 mL
(8) Shading bottle for storage


Fig. 4-2 What you need to prepare 2 mM Potassium ferrocyanide:
(1) Potassium ferrocyanide
(2) Spatula
(3) Beaker 50 mL
(4) Metric cylinder 100 mL
(5) Glass rod (stirring rod)
(6) Volumetric flask
(7) Pipette 5 mL
(8) Shading bottle for storager


Fig. 5-1 Weigh the potassium ferricyanide (1) using directly the beaker 50 mL (2) with an analytical digital balance.


Fig. 5-2 Weigh the potassium ferrocyanide (1) using directly the beaker 50 mL (2) with an analytical digital balance.


Fig. 6-1 To dissolve the potassium ferricyanide, use the 1 M Potassium nitrate solution prepared in the previous section. The metric cylinder is not required, it was used to know an approximately volume and to prevent the spilling.


Fig. 6-2 To dissolve the potassium ferrocyanide, use the 1 M Potassium nitrate solution prepared in the previous section. The metric cylinder is not required, it was used to know an approximately volume and to prevent the spilling.


Fig. 7 Add 1 M potassium nitrate solution to the beaker 50 mL (3) with potassium ferricyanide that has been previously weighed. Mix with the glass rod until it dissolves and transfer to the flask draining the solution on the glass rod to prevent the solution from being spilled. Repeat the same process for the potassium ferrocyanide.


Fig. 8 Transfer the prepared and adjusted solution in to the potassium nitrate in to the Shading bottle for storage (8). Do the same for both solution.
- Measure the 0.0658 g of the potassium ferricyanide and 0.0844 g of the potassium ferrocyanide directly in the beaker 50 mL, using an analytical digital balance (Fig. 5-1 and 5-2).
- Fill the metric cylinder 100 mL with 1 M Potassium nitrate solution prepared in a previous section. The metric cylinder is not required, because the 100 mL volumetric flask will be used and at the end the volume will be adjusted with a pipette. Here, the metric cylinder was used to separate the quantity and to prevent the solution to being spilled.
- Add small quantity of 1 M potassium nitrate solution in the beaker and dissolve the salt mixing with a glass rod.
- Transfer the solution to the flask draining the solution on the glass rod to prevent the solution from being spilled
- Rinse the beaker with 1 M Potassium nitrate and transfer the liquid to the volumetric flask, repeat some times this process.
- Adjust the volume to 100 mL, adding 1 M Potassium nitrate solution with a pipette.
- Transfer the solution from the volumetric flask to the Shading bottle for storage.

