Part 16: Bulk Electrolysis (BE)
The principle of bulk electrolysis (BE) is quite simple. If only oxidized molecular species are initially present, the potential is set to a negative value sufficient to cause rapid reduction and is maintained at this value until only reduced species are present in solution. According to Faraday's law, the total charge (Q) passed during the BE experiment is related to the number of moles (N) of the initially present molecules of the oxidized state of the substance and the number of electron transfers (n) of the individual molecules, where F is Faraday's constant (96,500 C/mol). Thus, if either n or N is known, one can be calculated.

Fig. 16-1 Potential waveform of BE.
The cells required for BE are different from those needed for voltammetry experiments (only a small fraction of the electrochemically active molecule of interest is electrolyzed in voltammetry). The rate of electrolysis is increased by using a working electrode with a large surface area (e.g., platinum wire mesh or mercury pool) and a counter electrode with a large surface area (e.g., platinum coil or wire mesh).
The solution is agitated to increase the rate of mass transfer in and out of the working electrode. The counter electrode is isolated from the working electrode to prevent interference between electrolysis products at the counter electrode and electrolytic species at the working electrode. Care must be taken in selecting the material that isolates the working electrode from the counter electrode. This is because high electrical resistance of the material may affect the efficiency of the electrolysis.
Prior to the BE experiment, select the potential. For reduction, the ideal potential should be about -200 mV below the redox potential (measured, for example, by cyclic voltammetry). The rate of electrolysis is governed by the rate of mass transfer to the working electrode. However, if the electrolytic potentials of other electrochemically active substances (e.g., electrolytes, solvents, other components in solution) are close, a potential that is not very far from the redox potential may not be used.
During the BE experiment, the clock on the PC monitor will display the experimental time. The average current for the first interval and the average current ratio for each interval are also displayed. This ratio is an important criterion for determining the degree of electrolysis. In general, electrolysis is considered to be finished when the ratio reaches 1% (the residual current, which is the background current). The final current ratio can also be set by the user (1% is the default value). The results are displayed in a charge vs. time plot (Fig. 16-2) or current vs. time plot (Fig. 16-3).
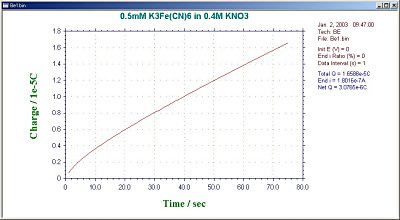
Fig. 16-2 Charge vs. time plot for a typical BE.
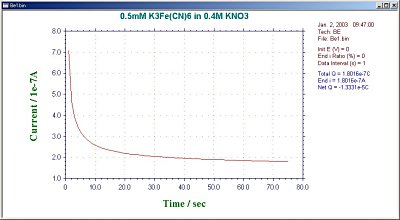
Fig. 16-3 Current vs. time plot of a typical BE.

