Part 6: Conversion stripping method
A self-induced redox cycle can be generated even if the redox substance in the cell housing the macroelectrode is replaced by a precipitable one. In particular, the use of heavy metal and halogen ions, whose precipitation and dissolution can be controlled by the potential of the electrode, enables the conversion and integration of the detected current as shown below.[16,17]
When the sample is placed in the cell on the IDA side, the depositable material is placed in the cell on the macroelectrode side, and the electrolysis (pre-electrolysis) of the sample is performed by controlling the potential of one side of the IDA electrodes (Fig. 6-1 (b)), a deposition reaction is induced at the macroelectrode due to self-induced redox cycling. The deposition is equal to the amount of electrolysis of the sample at the IDA electrode, according to the law of conservation of electric charge.
In other words, the detection current of the sample could be converted into a deposition current of another substance. Furthermore, prolonged electrolysis of the sample results in a large amount of deposition, since the deposition reaction continues at the macroelectrode as long as the electrolysis of the sample continues. This means that the detected current could be time integrated. The amount of material deposited on the macroelectrode can be quantified by the conventional stripping method (Fig. 6-1 (c)).
This is done by measuring the current peak obtained when the potential of the macroelectrode is swept. From the peak area, the total deposition and thus the total amount of electrolysis of the sample can be quantified. Assuming that a constant current is flowing during pre-electrolysis of the sample, the concentration of the sample can be determined from the limiting current values determined by the total amount of electrolysis, the pre-electrolysis time and the shape of the IDA electrode.
This analysis method converts the detected current of the sample into the deposition current of another substance, and the deosited substance is subjected to normal stripping analysis, allows the determination of reversible redox substances at very low concentrations due to a two-step current amplification. That is, the first current amplification by redox cycle and the second amplification effect by time integration of deposited substances.
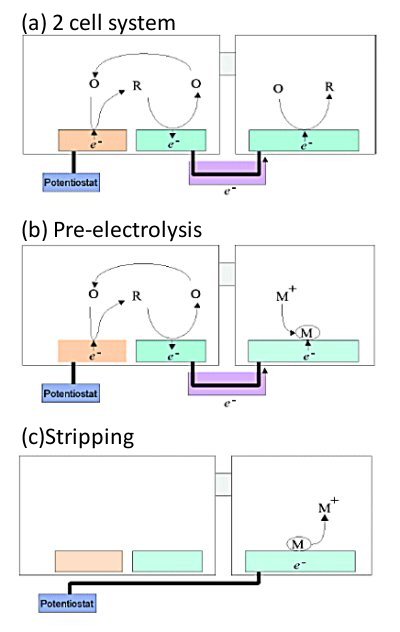
The cell was separated into two and electrically connected between them by a salt bridge. IDA electrode was placed in the left cell and a macroelectrode in the right cell.
(a) A self-induced redox cycle is also expressed in the two-cell system, and the current flowing between the IDA shaped electrode and the macro-electrode can be observed.
(b) Even if the redox substance in the right cell is changed to a substance that deposits on the electrode, such as heavy metal ions, the self-induced redox cycle still occurs, and the amount of reaction in the left cell is equal to the amount of deposition in the right cell.
(c) By controlling the potential of the macroelectrode, the heavy metal ions deposited in (b) can be dissolved. The amount of dissolution is equal to the amount of reaction performed in the left cell.

