Part 7: Application
7.1 Measurement of oxidized standard substances
As an example of the measurement of conversion stripping, the detection of ruthenium hexamine is described. Ruthenium hexamine is a reversible redox substance dissolved in an oxidized state and is suitable as a standard substance for the conversion stripping method.
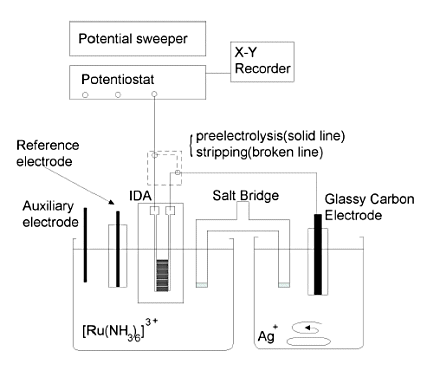
Fig. 7-1 shows a block diagram of the measurement system.
The measurement cell consisted of a Teflon beaker connected by a salt bridge. The salt bridge was a glass tube filled with saturated potassium nitrate solution and sealed at both ends with Vycor glass. IDA, Ag/AgCl reference electrode, and Pt counter electrode were placed in the left cell for the sample, and a glassy carbon (GC) electrode was placed as a macroelectrode in the right cell for the deposited material. The reference and counter electrodes were connected to the specified terminals of the potentiostat, while the two electrode terminals of the IDA electrode and the GC electrode terminal were connected to the working electrode terminal of the potentiostat through a switch box.
This switch box is used to switch between the pre-electrolysis and stripping phases. In the pre-electrolysis phase, one IDA working electrode (generator) is connected to the potentiostat and the other working electrode (collector) is connected to the macroelectrode, while in the stripping phase, the circuit is set up to connect the macroelectrode to the potentiostat.
The samples were prepared by dissolving ruthenium hexamine in pH 4 standard buffer solutions. The solution for deposition was silver nitrate dissolved in potassium nitrate solution (0.1 mol/dm3). The detection of ruthenium hexamine by the conversion stripping method was performed in two steps as follows.
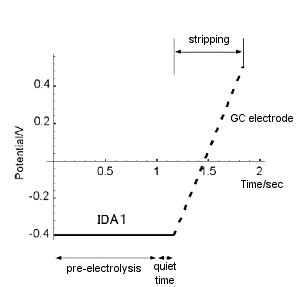
As shown in Fig. 7-2, during the first pre-electrolysis step, the generator potential was set sufficiently lower than the redox potential of ruthenium hexamine (-0.4 V in the figure) and the electrolysis of ruthenium hexamine was continued. During this time, the silver nitrate solution was continuously stirred. After a preset time (pre-electrolysis time), the stirring was stopped, and after 10 sec of quiet time, the switch was immediately switched to perform the second step of stripping. During stripping, the GC electrode potential was swept from -0.4 V to 0.5 V at a scan rate of 20 mV/S. The potential-current curve of the GC electrode (conversion stripping voltammogram) was recorded on a recorder. The obtained conversion stripping voltammogram of ruthenium hexamine is shown in Fig. 7-3.
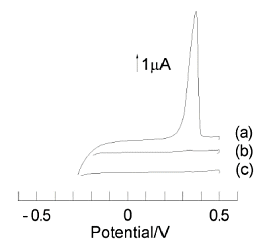
(a) Pre-electrolysis of a solution containing ruthenium hexamine at -0.4 V for 10 min.
(b) Pre-electrolysis of a solution containing ruthenium hexamine at 0 V for 10 min.
(c) Pre-electrolysis of a solution without ruthenium hexamine at -0.4 V for 10 min.
All potential sweeps were 20 mV/sec
The concentration of ruthenium hexamine is 1 µmol/dm3. (a) was a stripping voltammogram of silver ions obtained after 10 min of pre-electrolysis of ruthenium hexamine at -0.4 V. In contrast, (b) was obtained after pre-electrolysis at 0 V for 10 min. (c) was obtained after 10 min of pre-electrolysis at -0.4 V in a solution without ruthenium hexamine.
A large silver stripping peak was observed only when ruthenium hexamine was present and the electrolysis was being performed. A recorder was inserted between the collector and the GC electrode to monitor the current flowing during the pre-electrolysis step, and it was confirmed that a steady state was reached about 30 sec after the start of pre-electrolysis.
From the above, it is clear that in the conversion stripping method, ruthenium hexamine in the left cell is reduced by the generator, the reduced product generated is oxidized by the collector, and the electrons generated by the oxidation reaction are used to deposit silver ions in the right cell. The electrons generated by the oxidation reaction are used for the deposition of silver ions in the right cell. The oxidized product generated in the collector is again reduced in the generator to form a self-induced redox cycle, and the current flowing between the collector and GC is found to be steady-state.
Therefore, the amount of silver deposited is proportional to the pre-electrolysis time, indicating that a long pre-electrolysis time is effective for detecting low concentrations.
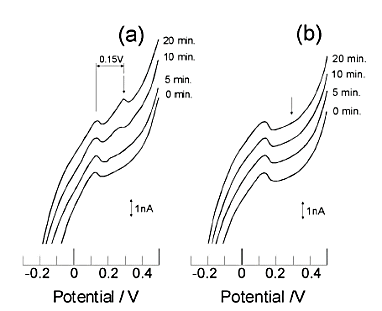
(a) Measured in solution containing ruthenium hexamine.
(b) measured in solution without ruthenium hexamine.
The number to the right of each voltammogram indicates the pre-electrolysis time.
A peak at 0.3 V indicates the detection of ruthenium hexamine.
Fig. 7-4 shows the results of applying conversion stripping to 10 pmol/dm3 of ruthenium hexamine[18]. In order to observe a smaller stripping peak, the conditions such as GC electrode area and silver ion concentration are different from those in Fig. 7-3 (a) is a voltammogram obtained from a solution containing ruthenium hexamine and (b) is a voltammogram obtained from a solution without ruthenium hexamine. A pre-electrolysis time-dependent stripping peak was observed at 0.3 V, in (a), whereas no corresponding peak was observed in (b). Also, Fig. 7-5 shows the calibration curve for the conversion stripping method[19].
The horizontal axis is the concentration of ruthenium hexamine and the vertical axis is the magnitude of the stripping peak obtained at a pre-electrolysis time of 10 min. The detection method showed good linearity in thep mol/dm3 region. The solid line in the figure is the calculated value obtained from the limit current [14] and peak width of the IDA electrode, which is in good agreement with the experimental value.
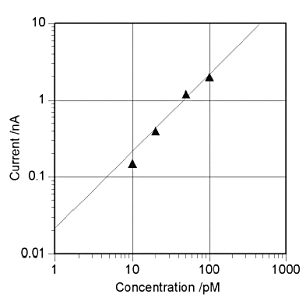
Pre-electrolysis time is 10 min. Solid line is calculated value.
The conversion stripping method can obtain a very large detection current compared to that obtained by the normal electrochemical detection method. Although the IDA electrode used in the conversion stripping method cannot detect 10 pmol/dm3 of ruthenium hexamine using the normal electrochemical detection method, the limiting current value in the twin mode is 0.8 pA when evaluated using calculated values[5]. In contrast, the measured value of the peak obtained after 20 minutes of pre-electrolysis by the conversion stripping method is 0.5 nA, which means that a detection signal 630 times higher was obtained. Furthermore, in the single electrode, where the redox cycle effect is not available, the amplification effect is even greater because the detected current is as low as 10%.

