Part 7: Application
7.2 Measurement of reduced standard samples
So far, we have described examples of applying conversion stripping to redox substances dissolved in an oxidized state, but the conversion stripping method can also be applied to reversible redox substances dissolved in a reduced state. The results of applying a water-soluble ferrocene suitable for conversion stripping as a standard dissolved in the reduced state are described below.
The mechanism and method of detection of water-soluble ferrocene by the conversion stripping method is similar to that for ruthenium hexamine. The difference is that the oxidation and reduction reactions are reversed, and accordingly, the direction of electrons is also reversed. As shown in Fig. 7-6, in the pre-electrolysis stage, the oxidation reaction occurs in the generator and the reduction reaction occurs in the collector, and the electrons required for the reduction reaction in the collector must be supplied from the macroelectrode.
This is possible by choosing halogen ions as the substance to be deposited on the macroelectrode. In the experiment, iodine ions with appropriate deposition potential were used. A silver electrode was used as the macroelectrode for depositing the iodine ions. In the stripping stage, the dissolution current of iodine ions is observed by observing the cathodic current (convex peak at the bottom) when the macroelectrode potential is swept from positive to negative.
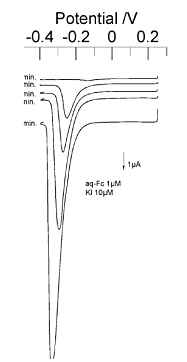
Fig. 7-6 shows an example of water-soluble ferrocene detected by the conversion stripping method. The concentration of water-soluble ferrocene was 1 µmol/dm3 dissolved in a phosphate buffer solution at pH 7.0.
The solution for deposition was potassium iodide dissolved in 0.1 mmol/dm3 potassium nitrate solution with a concentration of 1 µmol/dm3. A large peak of cathodic current was observed depending on the pre-electrolysis time, whereas this peak was not observed in the experiment without water-soluble ferrocene.
From this, it is confirmed that iodine ions are deposited on the macroelectrode during the electrolysis of water-soluble ferrocene, and the deposited iodine ions dissolve in the stripping step.The law was shown to be effective.
So far, we have seen the possibility of detecting trace substances using the conversion stripping method as a quantitative analysis technique, but the conversion stripping method also has the possibility of being a qualitative analysis technique for trace substances.
Fig. 7-7 plots the stripping peaks obtained for the conversion stripping of water-soluble ferrocene as a function of pre-electrolytic potential. When the pre-electrolysis potential is lower than the redox potential of water-soluble ferrocene, no stripping peak appears, but as the pre-electrolysis potential approaches the redox potential, the peak becomes larger, and at pre-electrolysis potentials sufficiently higher than the redox potential, the peak size is constant.
The measurements can be fitted with a sigmoidal function. The fitting parameter, which stands for the redox potential, was determined to be 0.38 V by the nonlinear least squares method, which agreed with the value of the redox potential of water-soluble ferrocene.
This measurement is equivalent to cyclic voltammetry with a IDA electrode in twin mode, but the detection current is amplified to a very large extent, indicating that cyclic voltammetry measurement of low concentration samples is possible.
However, this measurement requires a long measurement time because a pre-electrolysis must be performed for each point obtained. This can be solved by performing conversion stripping with an electrode system consisting of many pairs of IDA shaped electrodes and macroelectrodes.
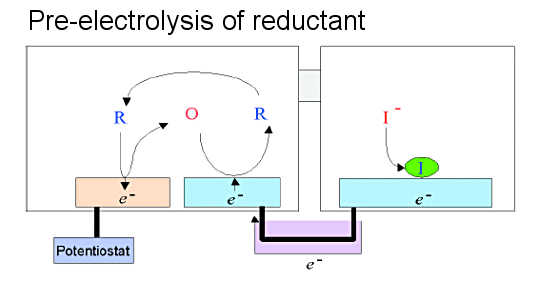
Fig. 7-6 Pre-electrolysis mechanism for a reversible redox substance dissolved in a reduced state. The oxidation and reduction reactions at each electrode are opposite when the substance is dissolved in an oxidized state. The direction of the current flowing between the IDA electrode and the macroelectrode is also opposite. The deposited substance is an anion.