Part 7: Application
7.3 Analysis of micro volume sample
In a redox cycle of reversible reactive species on an IDA electrode, most of the molecules reacted on one electrode are returned to their original state by a reverse reaction on the other electrode. Therefore, even when the amount of solution is extremaly small compared to the electrode area, the measurement can be performed stably without changing the concentration of reactive species in the sample due to electrochemical reactions. We fabricated a micro electrochemical cell of 2 mm square by integrating a reference electrode and a counter electrode on a IDA electrode. The reference electrode was made by plating silver on the electrodes other than the IDA electrode formed on the substrate. The IDA electrode cell was thinned to a volume of less than 1 microliter using a Teflon gasket and a glass plate.
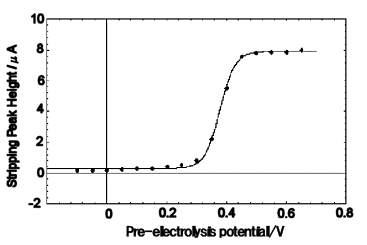
1µ mol/dm3 water-soluble ferrocene was pre-electrolyzed for 5 minutes by changing the pre-electrolysis potential.
Solid lines are fitted with sigmoidal functions.
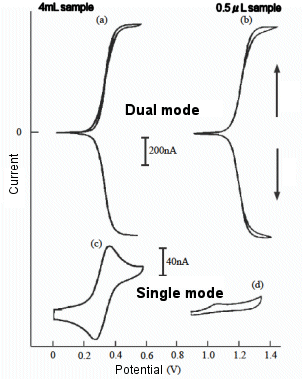
Fig. 7-9 shows the voltammograms of a ferrocene derivative solution with a sample volume of 500 nL measured in a micro IDA electrochemical cell, compared to the results obtained with a larger sample volume. When the reduction potential of ferrocene is applied to one electrode and the other electrode is swept, a large steady-state current is obtained even with a very small sample volume, but when only one electrode is used for measurement, the current value is very small, and this difference is more pronounced than when the sample volume is large. This result indicates that when only one electrode is used for measurement, the active species are rapidly consumed by the electrochemical reaction. On the other hand, when two electrodes are used for the measurement and the potentials are applied independently so that a redox cycle occurs, the micro IDA electrode has a high capture rate and the ferrocene derivative is oxidized at the G-pole and immediately reduced at the C-pole, thus reducing the consumption of the reductant.
The micro IDA electrode cell was used as a detector in an electrochemical enzyme immunoassay to measure para-aminophenol, a product of the enzymatic reaction. As a result, the same quantitative performance was obtained with a sample volume of less than a microliter as with a larger sample volume. It was also found that the measurable time was reached after a short incubation time (enzyme reaction time) due to the small solution volume [21].