Part 2: Effect of relative permittivity of the solvent (1)
The solvation energy of ions is related to several elements of the ion-solvent interaction, where the electrostatic solvation energy ΔGel can be expressed by the following Born formula[1]。
In equation (1), ze is the charge of the ion; r is the radius; N is Avogadro's constant; εr is the relative permittivity. Assuming that r is a constant in this equation, the absolute value of ΔGel decreases as εr decreases. However, the decrease in the absolute value of ΔGel is relatively flat in the range of εr greater than 20, but shows a sharp decrease in the range of εr less than 10. Since the electrostatic solvation energy ΔGel occupies most of the total solvation energy of ions, solvents with small relative dielectric constants (especially εr < 5) generally have weaker solventization of ions due to their electrostatic solvation energy ΔGel becoming smaller in absolute value.
In addition, the cations and anions in solution can exist in a dissociated state that allows the solution to conduct electricity, or they can combine as ion pairs, as shown in equation (2).

Assuming that the constant of the association reaction between the solvated cation and solvated anion is Kass, logKass can be roughly expressed by the following Fuoss approximation equation (3).
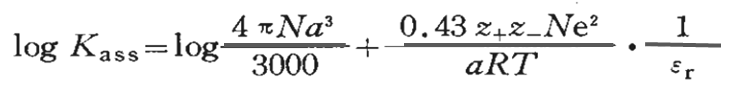
where z+e and z-e are the charges of M+ and X- and α is the closest distance between M+ and X- ions .
From equation (3), it can be seen that logKass is almost linearly related to the inverse of αεr, and it can be predicted that if the relative permittivity εr is smaller, or the nearest distance α between ions is smaller, the easier it is to form ion pairs whose solution conductivity is reduced.
The relationship between log Kass and εr inverse for tetrabutylammonium picrate with larger values of α is seen below, and a better linear relationship can be seen between them.
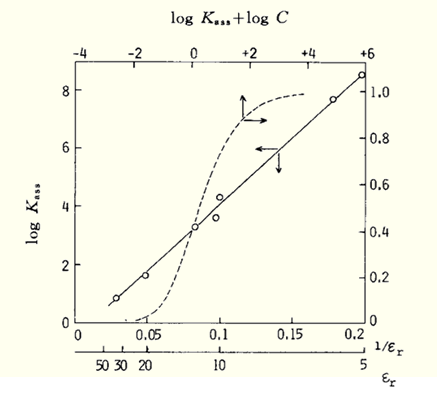
solvents: ➀ C6H5NO2, ➁ Ac, ➂ Py, ➃ CH2ClCH2Cl, ➄ CH3CHCl2, ➅ C6H5Cl, ➆ m-C6H4Cl2
(Y. H. Inami et al., J. Phys. Chem. 83, 4745 (1961).
The dashed line is the relationship between the degree of conformance α and log(C Kass ) (molar concentration of C: Bu4NPic mol dm-3).
Assuming that the analytical concentrations of the tetrabutylammonium cation and picrate anion in solution are C M (M = mol dm-3), there is a relationship between the degree of association α between M+ and X- and log(C Kass ) as shown by the dashed line in Fig. 2-1. For example, for 0.01 M tetrabutylammonium picrate, it can be seen that more than 80% dissociation occurs when the εr of the solvent used is >30, while more than 90% exists as ion pairs when the εr of the solvent is <10.
Reference
[1] A. J. Bard, ed., Electroanalytical Chemistry, Vol. 3, C. K. Mann, Non-aqueous solvents for electrochemical use, p. 57 (1969), Marcel Dekker.

