Part 4: Solvent donating and accepting properties and solvent classification
The electron (pair) donor and acceptor properties of a solvent, together with the relative permittivity are factors that affect many reactions and equilibrium that occur in solution. In particular, when comparing the solute behavior of various solvents with high relative permittivity used in the electrode reaction, the effects of the differences in the donor and acceptor properties of the solvents are more pronounced than those in the relative permittivity.
As a measure of the strength of solvent donating and accepting, the donor number (Donor number, expressed in DN) and acceptor number (Acceptor number, expressed in AN) advocated by Gutmann are widely used[4][5].
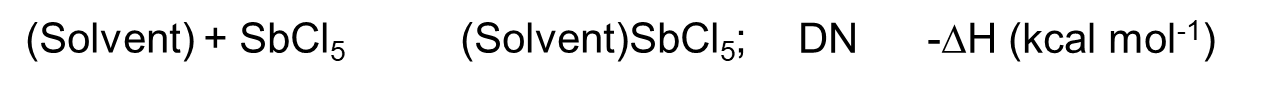
The method of measuring the number of donors is that in the dichloroethane of the weak donor, the sample solvent (donor) (Body) and SbCl5 (strong acceptor, stronger Lewis acid) occur as shown in equation (6). The heat of the product formed during the addition reaction is the number of donors. The larger the DN value, the stronger the donating property of the solvent.

On the other hand, the receptor number is a measure of the strength of the receptivity, and the receptor number is measured. If there is such an interaction between triethylphosphine oxide dissolved in the sample solvent (receptor) and the solvent, in which case the NMR low magnetic field shift δ of phosphorus 31 is measured using n-hexane as a reference, the receptor number AN in n-hexane is regarded as 0, and the receptor number AN (Acceptor number) at the time of the interaction between antimony pentachloride and triethylphosphine oxide in ethylene chloride is expressed as 100.
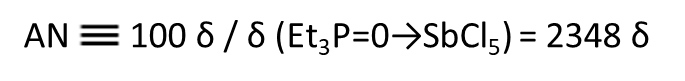
(7)
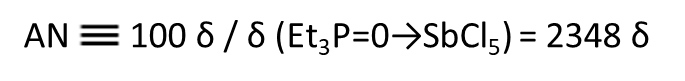
The acceptor number AN of the solvent can be calculated by Equation (7). A higher AN value indicates a stronger (electron) acceptability of the solvent.
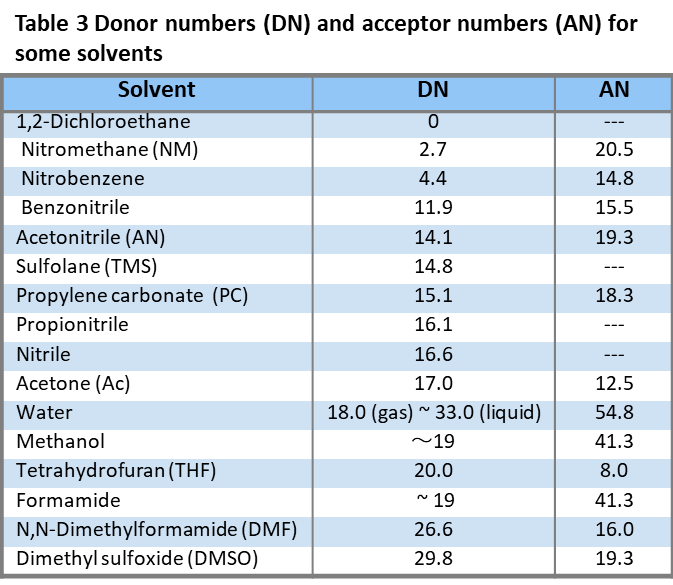



Table 3 shows the donor and acceptor numbers of some solvents in increasing order of donor number. The donor and acceptor numbers are the strengths of the interactions of the solvents as Lewis bases and Lewis acids with antimony pentachloride and triethylphosphine oxide, respectively, and they are also closely related to the strengths of the Bronsted bases and acids, respectively.Solvents with larger DN (which are more proton accepting and hydrogen bonding accepting) are more basic, while solvents with larger AN (which are more proton giving and hydrogen bonding) are more acidic. However, there is little correlation between the number of donors and acceptors of a solvent and the relative dielectric constant.
Solvents are usually classified in terms of Bronsted acids and bases, i.e., loss or gain of protons. An example of Prof. Kolthoff's classification of solvents is shown in Table 4. Amphoteric solvents are solvent molecules that are both acidic and basic. When the solvent is denoted by SH, the solvent molecule can release protons and can also receive protons in this way. Among the amphoteric solvents, those with acidic and basic strengths similar to those of water are neutral solvents.
Solvents that are much more acidic than water and much weaker in alkalinity, such as sulfuric acid, hydrofluoric acid, formic acid, acetic acid, etc., are proton donating solvents. On the other hand, solvents that are weaker than water in acidity and much stronger in alkalinity, such as ethylenediamine, NH3, formamide, etc., are protophilic solvents.
On the other hand, aprotic solvents generally have only hydrogen atoms bonded to carbon atoms, so they are weak in releasing protons and donating hydrogen bonds. However, there are strong and weak points in alkalinity. Strong bases are aprotic and weak bases are aprotic. Among aprotic solvents, solvents with large dipole moment and dielectric constant are polar aprotic solvents (dipolar aprotic solvents) and are usually used for electrode reactions.
This is because protons and hydrogen bonding are hardly involved in the reaction in these solvents, which makes possible many phenomena that are not possible in proton solvents such as water. Among the polar aprotonic solvents, AN, PC, TMS, NM, etc. are hydrophobic solvents with little affinity for protons, while these solvents of DMF, etc. are protonophilic. Acetonitrile (AN) Propylene Carbonate (PC) Sulfolane (TMS) Nitromethane (NM).
In reactions where the basicity (electron donating) of the solvent plays an important role, these two groups of solvents exhibit distinctly different properties, and thus need to be appropriately selected according to the purpose.
Reference
[4] V. Gutmann, "The Donor-Acceptor Approach to Molecular Interactions", (1978), Plenum Press.
[5] V. Gutmann, Electrochim. Acta, 21, 661 (1976).
[6] I. M. Kolthoff, Anal. Chem., 46, 1992 (1974).
[7] I. M. Kolthoff and P. J. Elving, ed., "Treatise on Analytical Chemistry", 2nd ed., Part I, Volume 2 (1979).
Solvents are usually classified in terms of Bronsted acids and bases, i.e., loss or gain of protons. An example of Prof. Kolthoff's classification of solvents is shown in Table 4. Amphoteric solvents are solvent molecules that are both acidic and basic. When the solvent is denoted by SH, the solvent molecule can release protons and can also receive protons in this way. Among the amphoteric solvents, those with acidic and basic strengths similar to those of water are neutral solvents.
Solvents that are much more acidic than water and much weaker in alkalinity, such as sulfuric acid, hydrofluoric acid, formic acid, acetic acid, etc., are proton donating solvents. On the other hand, solvents that are weaker than water in acidity and much stronger in alkalinity, such as ethylenediamine, NH3, formamide, etc., are protophilic solvents.
On the other hand, aprotic solvents generally have only hydrogen atoms bonded to carbon atoms, so they are weak in releasing protons and donating hydrogen bonds. However, there are strong and weak points in alkalinity. Strong bases are aprotic and weak bases are aprotic. Among aprotic solvents, solvents with large dipole moment and dielectric constant are polar aprotic solvents (dipolar aprotic solvents) and are usually used for electrode reactions.
This is because protons and hydrogen bonding are hardly involved in the reaction in these solvents, which makes possible many phenomena that are not possible in proton solvents such as water. Among the polar aprotonic solvents, AN, PC, TMS, NM, etc. are hydrophobic solvents with little affinity for protons, while these solvents of DMF, etc. are protonophilic. Acetonitrile (AN) Propylene Carbonate (PC) Sulfolane (TMS) Nitromethane (NM).
In reactions where the basicity (electron donating) of the solvent plays an important role, these two groups of solvents exhibit distinctly different properties, and thus need to be appropriately selected according to the purpose.
Reference
[4] V. Gutmann, "The Donor-Acceptor Approach to Molecular Interactions", (1978), Plenum Press.
[5] V. Gutmann, Electrochim. Acta, 21, 661 (1976).
[6] I. M. Kolthoff, Anal. Chem., 46, 1992 (1974).
[7] I. M. Kolthoff and P. J. Elving, ed., "Treatise on Analytical Chemistry", 2nd ed., Part I, Volume 2 (1979).

