Part 12: The effect of solvents on electrode reactions
The properties of the solvent, the properties of the supporting electrolyte, and changes in their behavior can have various effects on the electrode reaction.
Let’s first look at the effect of the solvent on the electrode reaction.
• Effects on the electrode reactions of metal ions and complexes
1) The potential of metal ions to be reduced to metal (or metal amalgam) will move towards the negative potential direction with the enhancement of the solvation of metal ions in the solvent, and there is a thermodynamic correspondence between the negative shift of this potential and the solvation energy. The effect of solvents on polarographic half-wave potentials is often used as a means of comparing the dissolution energy of metal ions.
2)Since the decomposition of solvents is not easy to occur in aprotic solvents, those alkaline earth metals, aluminum, zirconium and other ions that do not get a good polarogram due to hydrolysis in aqueous solutions can also obtain better reduction waves.
Reactive metals that react with water, such as alkali metals, can also be precipitated on the electrode in an aprotic solvent.
3) Low-valence metal ion complexes produced by electrode reactions can be stable in nonprotonic solvents.[13][14]
For example, the bipyridyl ligand ion of divalent iron, [Fe(bipy)3]2+, undergoes a 2-electron reduction in aqueous solution to produce a zero-valent iron complex in one step, however, in nonprotonic solvents it undergoes a 1-electron, 3-step, reversible reduction step to produce the more stable [Fe(bipy)3]- as below.
In aqueous solution:
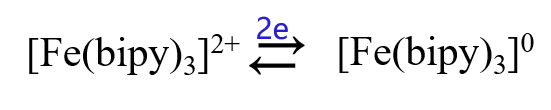
In nonprotonic solvents:
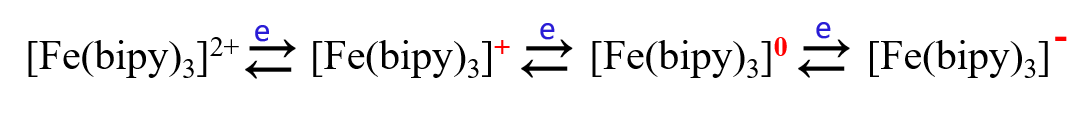
The same is true for various complexes such as Cr, Ni, and Ru bipyridine complexes.
This is because the aprotic solvent is weakly acidic and has difficulty reacting with the ligand (base) of the complex. From the perspective of obtaining potential reference standards that are independent of solvents, these reaction systems deserve everyone's attention.
In addition, since those complexes and organometallic compounds that are insoluble in water can usually be dissolved in organic solvents, these electrode reactions are studied in detail.
• Effect on the electrode reaction of organic compounds
Many organic compounds (R) are reduced by 2 electrons in aqueous solution to generate the hydrogenation reduction product RH2 in one step (Eq. 11).
However, in aprotic solvents, the mechanism of the reduction reaction is divided into two one-electron reduction steps. First, one electron is reduced to generate a fairly stable anionic radical, and then one electron is further reduced to R2- anion.
This phenomenon is very important in the history of the application of organic solvents to electrode reactions and has been extensively studied. Through research, it was found that as the number of solvent acceptors increases, the half-wave potential of anionic radicals generated by reduction in the first step will move forward linearly.
Aoyagi et al. used platinum electrodes in a DME solution of 0.4M tetrabutylammonium perchlorate to achieve four 1-electron reversible oxidation-reduction steps of cyclodecacyclic olefin molecules. And a relatively stable active anion equivalent to R4- was obtained in DME.
In addition, in polar aprotic solvents such as acetonitrile (AN), propylene carbonate (PC), nitromethane (NM), sulfolane (TMS), etc., it can be measured by using appropriate supporting electrolytes. To quite high positive potentials, even the oxidation of benzene, which is difficult to oxidize, can be observed.
The oxidation of organic compounds often generates in the first step a cationic radical (R ⇄ R+ + e), which is usually more unstable than the corresponding anionic radical. In protic solvents and aqueous solutions, these cationic radicals react with the solvent. However, if it is in an aprotic solvent, because there will be no reaction between the solvent and the cationic free radicals, free radical polymerization can occur.
Therefore, when measuring oxidation reactions, it is necessary to use a solvent with appropriate donor (basic) strength to achieve the target reaction.[15]
Reference
[13] T. Saji and S. Aoyagui, Inorg.Nucl. Chem. Lett., 2, 359 (1966).
[14] T. Saji and S. Aoyagui,Electrochim. Acta, 13, 335 (1968).
[15] T. Saji and S. Aoyagui, J. Electroanal. Chem., 102, 139 (1979).

