Part VI: Two meanings of equilibrium
(1) Equilibrium potential
The first meaning of equilibrium refers to the equilibrium potential, that is the potential difference between the individual half-cells when they are in equilibrium.
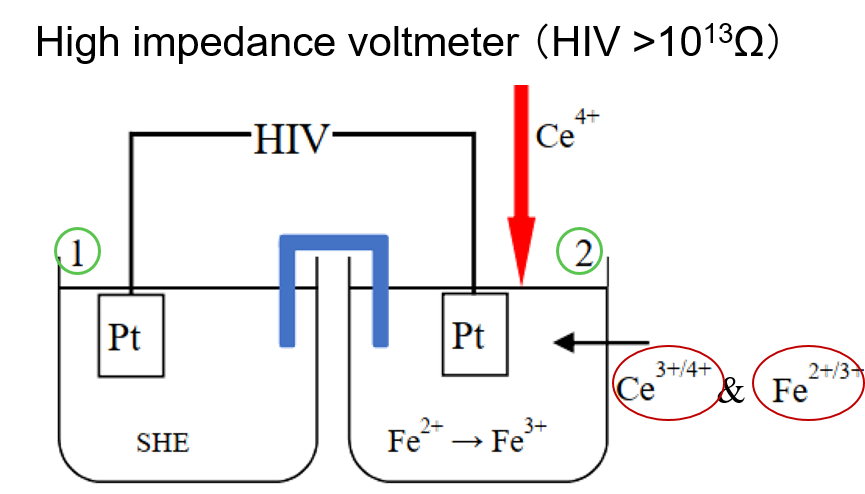
Fig. 6-1 Potentiometric titration of Fe2+ with Ce4+.
For the cell in Fig. 6-1, the standard hydrogen electrode is on the left and the titration reaction of Ce4+ with Fe2+ ions is on the right.
An example is the potentiometric titration of Ce4+ against Fe2+ ions: the half-cells (1), i.e., the standard hydrogen electrode, and (2) are connected with a high impedance potentiostat (HIV > 1013 Ω), and when the current is almost zero, it means that each of the half-cells is independently equilibrated at this point.
(2) Balance of battery voltage
The second level of balance is the balance of the cell voltage.
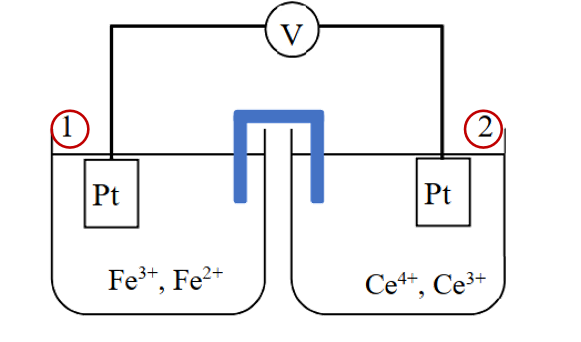
Fig. 6-2 Voltage of the battery.
For the battery configuration in Fig. 6-2, the battery voltage before use is: The potential difference between the two half-cells
The cell voltage after use becomes the potential difference between the two half-cells at equilibrium: Ecell = E2 - E1 = 0 V
The standard electrode potential of half-cell 1 is E10 and that of half-cell 2 is E20. The overall chemical reaction of the battery is that tetravalent Ce ions react with divalent iron ions to generate trivalent Ce ions and trivalent iron ions.
The Nernst equation for half cell 1 can be expressed as Eq. 39, and half cell 2 can be expressed as Eq. 40.
Half cell 1 Nernst eq.:
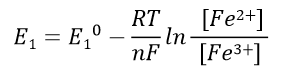
(39)
Half cell 2 Nernst eq.:
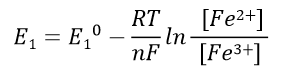
(40)
Battery voltage before the reaction: Expressed in equation 41, the battery voltage at the end of the reaction is 0 V. The relationship between the electric potential of the battery in the standard state and the equilibrium constant of the reaction, K, is expressed in reaction equation 42.
Battery electric potential before the reaction:
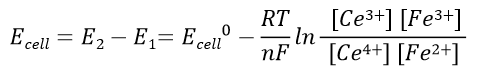
(41)
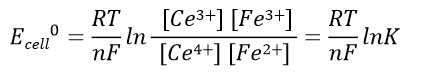
(42)
Electric potential of half-cell 1 and half-cell 2 in the standard state: Ecell0 = E20 - E10 which is the standard battery potential.
(to be continue...)

