ECSA Determining the 'Real' Performance of Electrocatalysts
M.Sc. Kimihiro Kinugasa
What is ECSA measurement?
ECSA measurement is a technique that uses electrochemical methods to determine the Electrochemical Surface Area (ECSA) of an electrode. In short, it is the effective area of the catalyst used in electrode reactions. This is a crucial indicator for evaluating noble metal catalysts like platinum and iridium used in fuel cells. The ECSA of noble metal catalysts is determined by dividing the charge (quantity of electricity) obtained from hydrogen adsorption/desorption waves or carbon monoxide (CO) stripping waves by the known value of adsorption charge per unit area of that noble metal. A larger value indicates better catalyst performance. It is also used to evaluate catalyst degradation behavior due to start-stop protocols ※1. On the other hand, for non-noble metals like nickel and their oxides, it is difficult to directly calculate ECSA because there is no suitable substance that adsorbs as a single layer on the electrode surface. As an alternative, "Electric double-layer capacitance (Cdl)" is sometimes used as an indirect indicator of ECSA.
※1: Refers to a potential cycle test that simulates the rapid potential changes during the start-up and shut-down of fuel cells.
How is ECSA measured?
A catalyst-supported electrode is used as the working electrode, and potential scanning (Cyclic Voltammetry: CV method) is performed. Hydrogen adsorption and CO desorption occur within specific potential ranges, so measurements are carried out within those ranges. However, the scanning potential varies depending on the pH and the type of catalyst. In all cases, the range is set to avoid side reactions such as water electrolysis.
For non-noble metal electrodes like nickel and their oxides, the potential range is set where no faradaic current (current due to electrochemical reactions) flows, allowing only charging and discharging of the electric double layer. Cdl is proportional to the surface area of the electrode in contact with the electrolyte. It's important to note that Cdl changes not only with surface area but also with the type of electrode material and electrode surface modification.
Measurement steps using a Model 3325 Bi-Potentiostat
In the example introduced here, a 3 mm diameter platinum electrode (RDE PTE Platinum disk electrode) for the RRDE-3A Rotating Ring Disk Electrode Apparatus Ver.3.0 is used to calculate the effective area of the electrode based on the amount of hydrogen adsorption. Although the platinum electrode appears smooth, a closer look reveals that due to the presence of scratches and other irregularities on the surface, its actual electrode surface area is slightly larger than the geometrically calculated area of 3 mm in diameter.
Procedure:
- Thoroughly polish the platinum working electrode (3 mm diameter) with an alumina polishing pad. Polishing time varies depending on individual pressure, but generally ranges from 2 to 4 minutes. After polishing, rinse thoroughly with distilled water. Do not use ultrasonic cleaning or wipe the surface with Kimwipes during this step.
- Use 0.5 M sulfuric acid as the electrolyte. Before measurement, purge the solution with inert gas (nitrogen or argon) to deoxygenate.
- Set the potential range for CV measurement. This time, using a silver/silver chloride reference electrode (Ag/AgCl) (RE-1B Reference electrode (Ag/AgCl)), the scanning range is -0.18 V to 1.2 V vs. Ag/AgCl.
(The Ag/AgCl reference electrode potential was previously calibrated using the H2G1 Portable Hydrogen Generator and RHEK Reversible hydrogen electrode kit, yielding EAg/AgCl vs. RHE = 0.196 V)
(After the experiment, the X-axis potential can be changed to the reversible hydrogen electrode (RHE) conversion potential.)
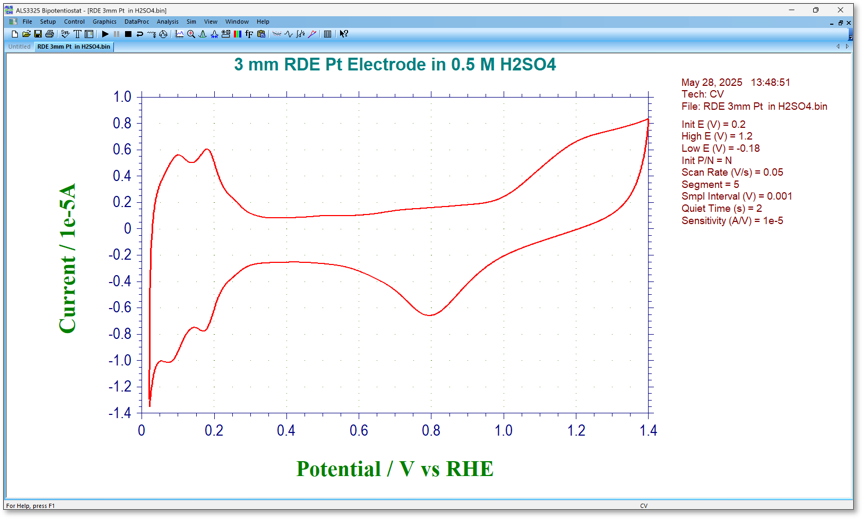
Fig. 1 Cyclic voltammogram of a platinum electrode in 0.5 M sulfuric acid solution
- Check the charge amount of hydrogen adsorption in the negative potential part.
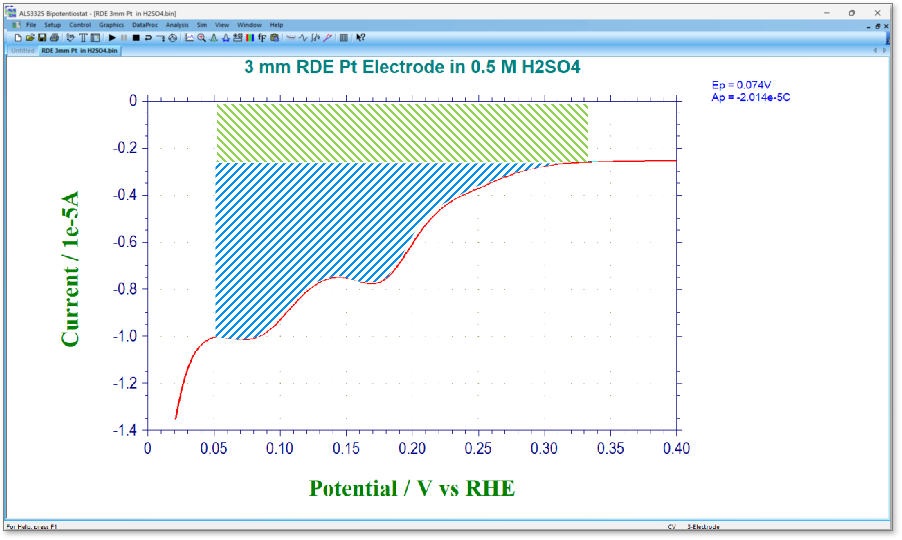
Fig. 2 Charge amount minus double layer capacitance (green diagonal line part) (blue diagonal line part: 20.14µC)
- According to the literature, the single-molecule adsorption amount of hydrogen on the electrode surface is 210 µC/cm2. Therefore, divide the charge amount confirmed in step 4 by this value to obtain the electrode active area. The charge amount in the blue shaded area is 20.14 µC, so the electrochemical effective area is 20.14 µC ÷ 210 µC/cm2 = 0.095 cm2 It can be seen that this value is about 1.3 times larger than the geometrically calculated effective area of 0.071 cm2 based on the electrode diameter of 3 mm.

