Part I: Electrode Potential Equilibrium and Electrode Reaction Kinetics (1)
♦ Balance electrode potential
When an electrochemical reaction system is in equilibrium, even if no apparent current flow is observed, this does not mean the reaction has completely ceased. From a kinetic perspective, this represents a state of dynamic equilibrium where the forward reaction rate equals the reverse reaction rate. Under these conditions, the terminal voltage (i.e., electromotive force) stabilizes at a constant value. This implies that the potentials of the two electrode pairs on either side remain fixed at a specific level. This potential is termed the equilibrium electrode potential, or simply the equilibrium potential.
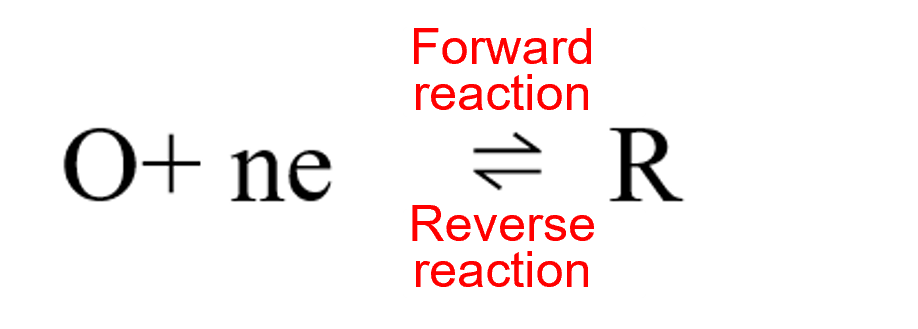
The equilibrium potential Eeq of a simple electron transfer reaction occurring at a single electrode (Equation 1) can be quantitatively expressed using the Nernst equation (Equation 2) based on the concentrations c (strictly speaking, the activities a) of the oxidized and reduced species present in the electrolyte solution.
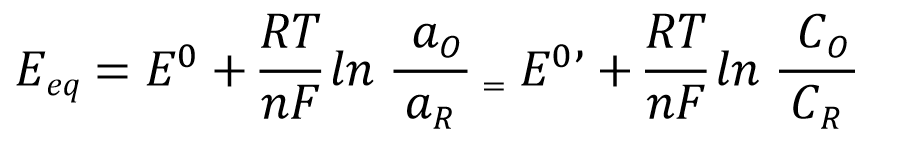
F:
R:
T:
E0:
E0':
Eeq:
Faraday's constant (96485 Cmol-1)
Ideal gas constant (8.31J K-1 mol-1)
Absolute temperature (K)
Standard electrode potential (standard electrode potential),
aR = aO = 1 (Standard temperature) Equilibrium potential at the time
Formal potential or conditional potential,
the standard electrode potential based on concentration.
Equilibrium potential Eeq (The experimental quantity that varies
according to the concentration ratio of redox substances participating in the reaction).
Therefore, the equilibrium potential Eeq is an experimental quantity that can vary depending on the concentration ratio of the redox species involved in the reaction. As previously explained, the electromotive force of a galvanic cell can be calculated using the Nernst equation from the quantitative relationships of these electroactive species.
♦ Electrode Reaction Kinetics (1): Polarization and Overpotential
When equilibrium is disrupted and the reaction begins to proceed in one direction, this direction can be reflected in the sign of the current (oxidation currents are positive, reduction currents are negative). For example, for reactions where oxidation is the positive reaction, the net reaction rate V can be expressed as the oxidation reaction rate minus the reduction reaction rate (Eq. 3).

Here, each reaction rate can be expressed as the product of the reactant concentration (mol cm-3) and the reaction rate constant k (cm s-1), just as in homogeneous reaction systems. Thus, the forward reaction rate Vox and the reverse reaction rate Vred can be represented by Eq. 4 and Eq. 5 below.
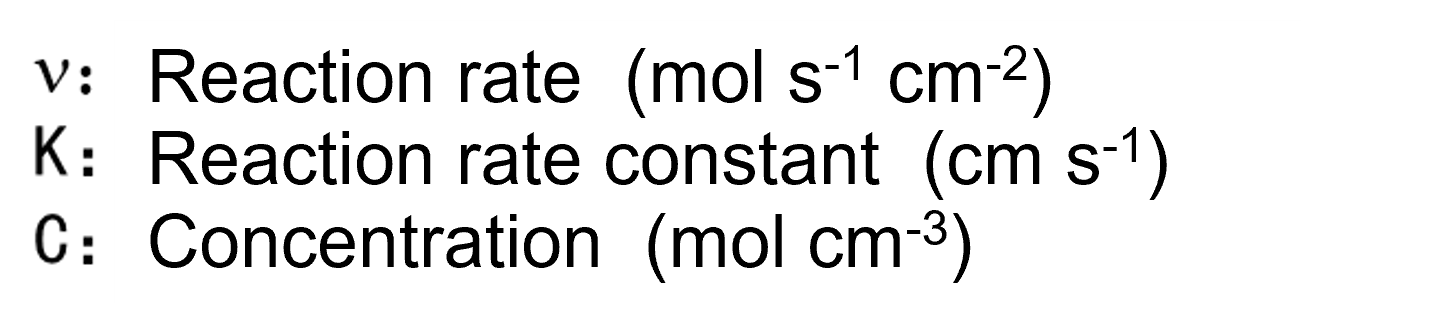
It should be noted that the CRs and COs appearing here do not refer to the concentration within the solution itself, but rather to the concentration at the interface between the electrode and the solution. This is hereafter referred to as surface concentration.
Because the absolute value of the current is directly proportional to the reaction rate. The oxidation-reduction current and reaction rate can be quantitatively related through Equations 6 and 7, and the observed net current I can be expressed as shown in Equation 8.
When a reaction occurs and current flows, the electrode potential begins to shift from its previous equilibrium value according to the direction and rate of the reaction. In other words, “the potential shift is a result of the reaction occurring,” but conversely, “forcing the potential away from its equilibrium value can enable the reaction to proceed.”
Potential or current—one of these can be used as a control variable and actively altered to track how the other quantity responds to this change, thereby providing a theoretical explanation. This constitutes the fundamental approach of electrochemical measurement methods.
Incidentally, both the operation of “shifting the potential from the equilibrium potential to induce a reaction” and the phenomenon of “potential shift caused by the reaction” are described using the electrochemical term “polarization.” (The amount of deviation from the equilibrium potential is referred to as overvoltage or overpotential.)
Its meaning is that “to generate a current of a certain magnitude, a voltage higher than the equilibrium potential is required.”
When the equilibrium potential is denoted by Eeq, the overvoltage (symbolized by the Greek letter eta) is the quantity defined by Eq. 9.
Where E is the electrode potential. Figure 1 shows the relationship between overvoltage and current.
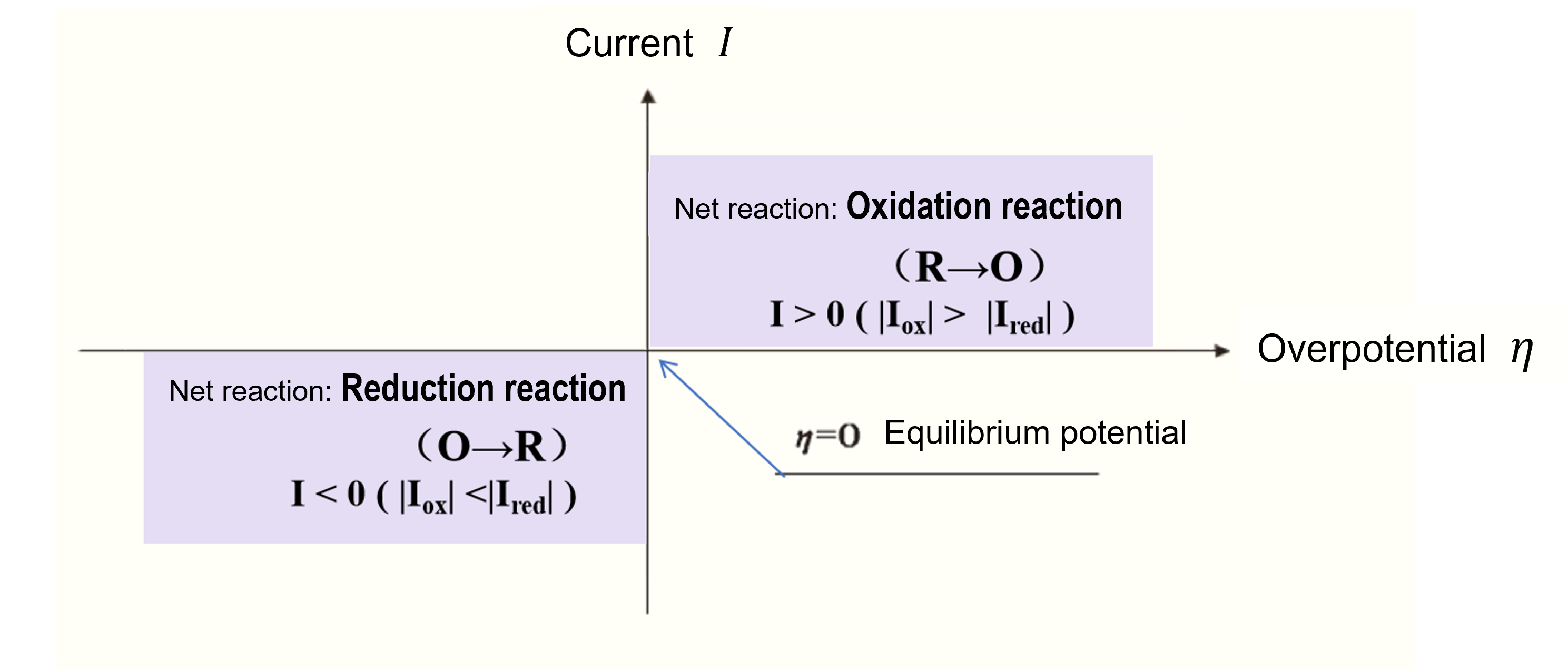
Fig. 1 Relationship between Overpotential (Overpotential) and Current.
In summary, whenever a non-zero current is observed, regardless of its magnitude, an overpotential (or overpotential) is generated.

