Reference electrodes
Reference electrodes are widely used as for electrochemical measurements (CV, LSV, DPV, etc.) and electrochemical devices (electrochemical detectors for HPLC, electrochemical biosensor, etc.). Various kinds of them such as aqueous, nonaqueous, calomel and own-constructing types are available.
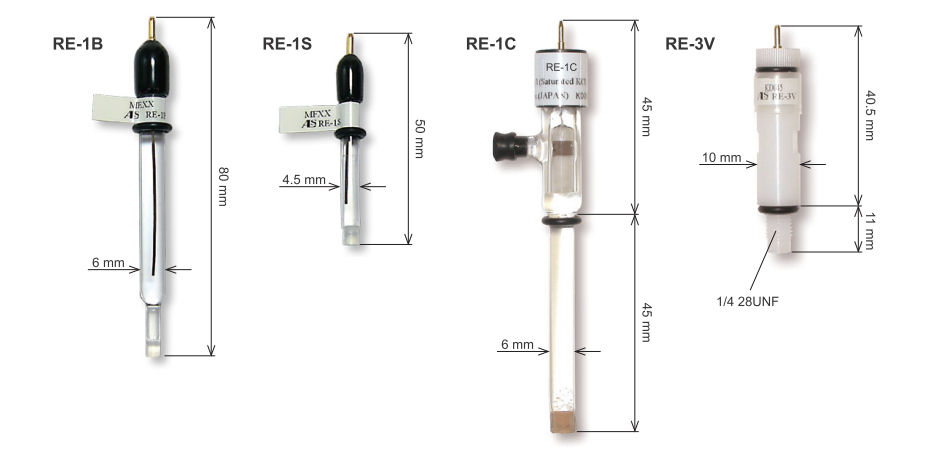
Catalog No. |
Description | Length (mm) | OD (mm) |
Liquid Conjunction Tip |
Internal solution |
| 012167 | RE-1B Reference electrode (Ag/AgCl) | 70 | 6 | Vycor glass | 3 M NaCl |
| 012168 | RE-1S Reference electrode (Ag/AgCl) | 40 | 4.5 | Vycor glass | 3 M NaCl |
| 012169 | RE-3V Reference electrode screw type (Ag/AgCl) | 48 | 10 | Vycor glass | 3 M NaCl |
| 012171 | RE-7 Non Aqueous reference electrode (Ag/Ag+) | 70 | 6 | Vycor glass | ACN/TBAP |
| 012172 | RE-7S Non Aqueous reference electrode (Ag/Ag+) | 40 | 4.5 | Vycor glass | ACN/TBAP |
| 012173 | RE-7V Non Aqueous reference electrode screw type (Ag/Ag+) | 48 | 10 | Vycor glass | ACN/TBAP |
| 012174 | RE-7VP Non Aqueous reference electrode screw type (Ag/Ag+) | 48 | 10 | Vycor glass | ACN/TBAP |
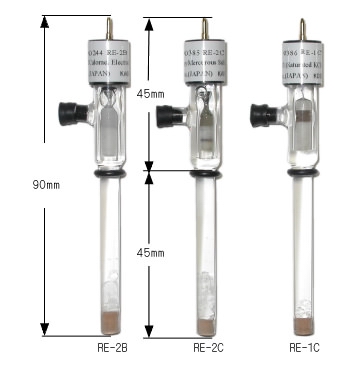
| 002058 | RE-1C Reference electrode (Ag/AgCl/Saturated KCl) | 90 | 6 | Ceramic | Saturated KCl |
| 002056 | RE-2B Calomel Reference electrode | 90 | 6 | Ceramic | Saturated KCl |
| 002057 | RE-2C Reference electrode | 90 | 6 | Ceramic | Saturated K2SO4 |
| 012974 | RE-6A Reference electrode for alkaline solution | 90 | 6 | Ceramic | -*- |
Applications
| RE-1B - Ag/Cl Reference electrode for CV RE-1S - Ag/Cl Reference electrode for SECM RE-1C - Ag/Cl Saturated KCl Reference electrode RE-3V - Ag/Cl Reference electrode applied to Flow cell RE-7 - Ag/Ag+ Reference electrode for CV of Non-Aqueous solvent RE-7S - Ag/Ag+ Reference electrode for SECM RE-7V - Ag/Ag+ Reference electrode applied to Flow cell RE-2B - Available as a standard electrode of reference electrodes RE-2C - Reference electrode free from chloride ion RE-6A - Reference electrode for alkaline solution |
Reference Electrode potentials at 25 °C
| NHE (Normal Hydrogen Electrode)--------------------------------------- 0 mV SCE (Potassium Saturated Calomel Electrode)---------------------- 241 mV SSCE (Sodium Saturated Calomel Electrode)----------------------- 236 mV Ag/AgCl (Saturated KCl)---------------------------------------------- 198 mV Hg/Hg2SO4 (0.5 M H2SO4)-------------------------------------------- 682 mV |
"Akira Fujishima, Masuo Aizawa, Toru Inoue, "Methods of Electrochemical Analysis (1984)" Gihodo shuppan"
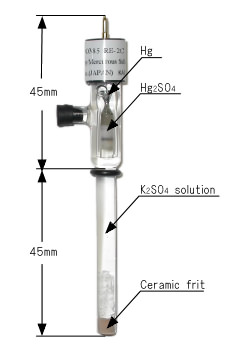
RE-2C Reference Electrode [Catalog No. 002057]
| Developed for the sake of eliminating any influence of chloride ion from measurements. Consists of mercury, mercury sulfate and saturated potassium sulfate solution. Hg2SO4 + 2e = 2Hg + SO42- E0 = 650 mV VS NHE (25 °C) |
 |
 |
|
| 002056 RE-2B Calomel Reference electrode | 002058 RE-1C Reference electrode (Ag/AgCl/Saturated KCl) |
| Hg2Cl2 + 2e = 2Hg + 2Cl- E0 = 241 mV VS NHE (25 °C) |
AgCl + e = Ag+ + Cl- E0 = 199 mV VS NHE (25 °C) |
| RE-2B Calomel Reference electrode and RE-1C Reference electrode (Ag/AgCl/Saturated KCl) have the same shape as RE-2C. | |
Technical note
Supporting electrolyte
If the sample is dissolved in an organic solvent, the supporting electrolyte must be added.
In order to select the supporting electrolyte, it is necessary to consider the following:
1. Solubility in organic solvents
2. Wide potential window
3. No reaction with organic solvent
Typical supporting electrolyte:
TEAP: Tetraethylammonium perchlorate
TBAPF6: Tetrabutylammonium hexafluorophosphate

