SEC2000 - Spectroelectrochemical cell
Spectroelectrochemistry (SEC) is aimed at the investigation of electrochemical reaction mechanism and the interface structure between electrolyte solution and electrode. Remarkable progress in this field and related technology enables SEC to be applied in wide areas. Nowadays, the relation between absorbance and potential for reversible or quasi-reversible system is theoretically elucidated, on which basis the analysis of electrochemical characteristics becomes possible for the system otherwise difficult with only the result of voltammogram. Typical example is redox enzyme cytochrome c and methylene-blue.
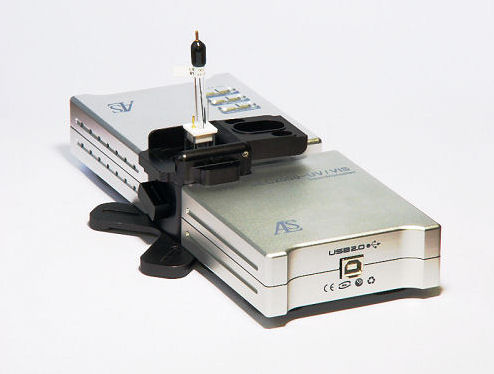
SEC2000-UV/VIS Spectrometer
SEC2000-UV/VIS Spectrometer is specially designed for spectroelectrochemical measurements. In the light source, the lens was incorporated to the light in a small module, then the fiber optics needed is eliminated.
SEC-C Spectroelectrochemical Cell
| Catalog No. | Description | ||
| 012904 | SEC-C Thin Layer Quartz Glass Spectroelectrochemical cell Kit | ||
| Components | |||
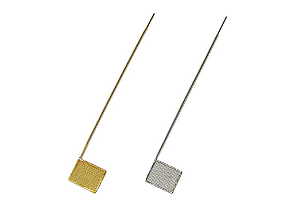
| 011498 | SEC-C Pt Gauze working electrode | ||
| 012906 | SEC-C Pt counter electrode | ||
| 012907 | SEC-C Thin Layer Quartz Glass cell | Light pass length: 1 mm | |
| 011501 | SEC-C Teflon Cap | ||
| 010537 | Purging tube | ||
| Optional products | |||
| 012167 | RE-1B Reference electrode (Ag/AgCl) | Length: 80.0 mm | OD: 6.0 mm |
| 012171 | RE-7 Non Aqueous reference electrode (Ag/Ag+) | Length: 80.0 mm | OD: 6.0 mm |
| 012017 | SEC-C Au Gauze working electrode | ||
| 011498 | SEC-C Pt Gauze working electrode | ||
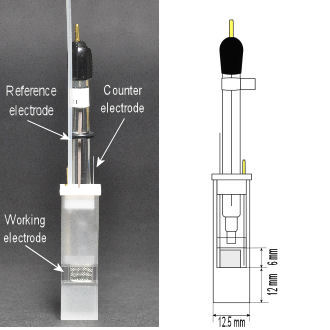
 Configuration of SEC-C Spectroelectrochemical cell Working electrode
Configuration of SEC-C Spectroelectrochemical cell Working electrode
Example of the simulation using Cuvette Spectroelectrochemical cell
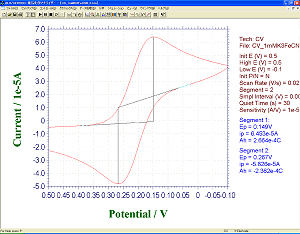
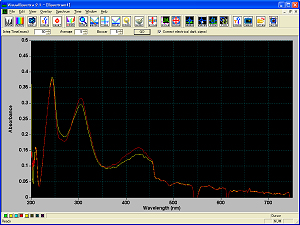
Absorption spectrum of visible ultraviolet or absorbance of the material involved in a reaction using Optically Transparent Electrode (OTE) was measured. Platinum or Gold gauze mesh electrode (light passes through the mesh open) was used. Absorbance (Fig. 1b) during CV measurement (Fig. 1a), and oxi-red phase condition (Fig. 2a, 2b) was observed for 1 mM Potassium Ferricyanide (K3[Fe(CN)6]) solution in a SEC-C Thin Layer Quartz Glass Spectroelectrochemical cell.
 |
 |
|
| Figure 1a. Cyclic Voltammogram of 1 mM K3[Fe(CN)6]. |
Figure 1b. Absorbance measurement of 1 mM K3[Fe(CN)6]. |
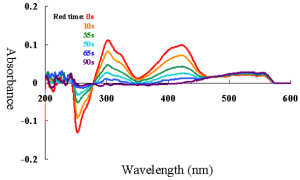
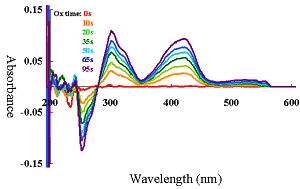
Spectroelectrochemical with CV simultaneous measurement, as well as with constant potential electrolysis was measured. Absorbance changes for oxi-red phase of Potassium Ferricyanide solution was shown in Fig 2a and 2b.
 |
 |
|
| Figure 2a. Reduction phase. | Figure 2b. Oxidation phase. |
SEC2000 - Combo kit
| Catalog No. | Description |
| 013253 | SEC2000-UV/VIS Spectrometer combo Kit Ver.1.3 |
| Components | |
| 013256 | SEC2000-UV/VIS Spectrometer Ver1.3 SLIT50 |
| 012193 | SEC2000-DH UV Light Source |
| 012195 | SEC2000-CUV Cuvette Holder |
| Catalog No. | Description |
| 013250 | SEC2000-VIS/NIR Spectrometer combo Kit Ver.1.3 |
| Components | |
| 013251 | SEC2000-VIS/NIR Spectrometer Ver.1.3 SLIT50 |
| 012194 | SEC2000-TH Visible Light Source |
| 012244 | SEC2000-CUV Cuvette Holder with diffusion plate |

