This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
5. Non-aqueous solvent
Professor Noriyuki Watanabe
In this section, we describe about a reference standard applicable for usage in organic solvent. The reference electrodes already mentioned for aqueous solvent are also usable in organic solvent by placing an appropriate salt bridge between test solution and aqueous reference electrode. As a matter of fact, the description of redox potential referred to SCE or Ag-AgCl is found in a lot of literature. In many of them, junction potentials between aqueous reference electrode and organic test solution have been ignored. So it is not always an easy task to compare our results with literature value or to evaluate discrepancy among literatures. Hence, considering inevitable contamination due to water and/or chloride ion and involvement of extra unaccountable junction potential, it might be better not to use aqueous reference electrode for non-aqueous study.
Ag+-Ag electrode is used in experiment for non-aqueous solvent in general. The basic structure of electrode is similar to Ag-AgCl electrode. Silver wire is inserted into a glass tube containing small amount of silver salt, organic solvent and large amount of electrolyte. Acetonitrile is employed as an internal solvent in most cases. The solvent used for an analyte solution is also usable in many cases. The electrolyte is selected from the combination of tetraalkylammonium R4N+ (R=methyl, ethyl, propyl and butyl etc.) as cation and perchlorate ClO4-, hexafluorophosphate PF6- and tetrafluoroborate BF4- so on as anion.
It is better for the larger concentration of electrolyte to be used as much as possible within solubility limit (typically more than 0.1 M). In addition to that, small amount of silver salt (0.01 M, the salt with NO3-, ClO4-, PF6- and BF4- etc.) is solubilized as Ag+ source. The long-term stability and reproducibility of this electrode is not always good. In addition to that, the reference potential depends on kinds of solvent. So relying upon Ag-Ag+ electrode only is not sufficient for the specification of redox potential of test compound. IUPAC (International Union of Pure and Applied Chemistry) recommends to use the internal standard such as ferrocene or bisphenyl chrome(I). The redox potential of these compounds does not depend so much on the kinds of using solvent. The reason is inferred that the redox site of Fe2+/3+ in ferrocene or Cr+1/0 in bisphenylchrome(I) are efficiently screened from influence of surrounding medium due to as sandwiched by pentadienyl ring or phenyl ring, respectively.
Even when you use Ag-Ag+ reference electrode, you should refer to the redox potential of ferrocene or bisphenylchrome as conventional potential standard. Bisphenylchrome(I) is used as tetraphenylborate salt. The redox potential of ferrocene is about +0.7 V on SHE scale, redox potential of bisphenylchrome(I) is -1.15 V on ferrocene scale (about -0.45 V on SHE scale). Both simultaneous measurements by as-contained in test solution or post-measurement by after-addition are viable. The virtue of employing internal standard is that a relative comparison with the potential on SHE scale becomes feasible via ferrocene scale.
Although the redox potential of Ag-Ag+ (Ag+ + e- ⇔ Ag) in aqueous solution is known as 0.7991 V vs SHE, this value changes largely in organic solvent due to difference of solvation energy toward mainly cation. For example, it is 0.65 V in dichloromethane, 0.41 V in tetrahydrofuran, 0.04 V in acetonitrile. As the oxidant (cation) is stabilized in polar solvent, the redox potential shifts toward negative direction depending on the extent of destabilization by the kind of solvent. Thus, non-aqueous Ag-Ag+ reference electrode might be not appropriate for specification of redox potential of analyte compound and just used as reference electrode. It might be recommendable that redox potential should be referred to the internal standard in your paper. Dimethylformamid(DMF) and dichloromethane are inappropriate from the view point of reactivity or solubility of Ag+ ion.
Pseudo-reference electrode is a way to be considered. It is just to connect Pt wire immersed in test solution to reference terminal of potentiostat (Because potentiostat has a reference electrode terminal which must be connected) which must not remain to be open. Although the specification of potential is not guaranteed, Pt wire might be a choice, considering low impedance of it and elimination of junction potential. Of course, it is needed to describe about the internal reference used in the case for publication.
How to use the non aqueous reference electrode
When non aqueous reference electrode is immersed directly into the sample solution, supporting electrolyte of the reference electrode internal solution, present in the IPPG (Ion Permeability Porous Glass) porous, could be dissolved in the sample or reacts with the measured sample, and it could be the reason of the clogging of the porous. Using the sample holder, as shown in the example below, it will prevent that it happened.
Fill the sample holder with an electrolyte solution, and check that there is no air bubble over the IPPG liquid junction. If there is some bubble, flick to take them out. The presence of the bubble, makes that the ion permeability will differ, and it could change the applied potential.
Then, set the non aqueous reference electrode into the sample holder. Set the position of the reference electrode moving the o-ring. Filling the sample holder with the same supporting electrolyte solution, as the reference electrode, the applied potential will be kept constant.
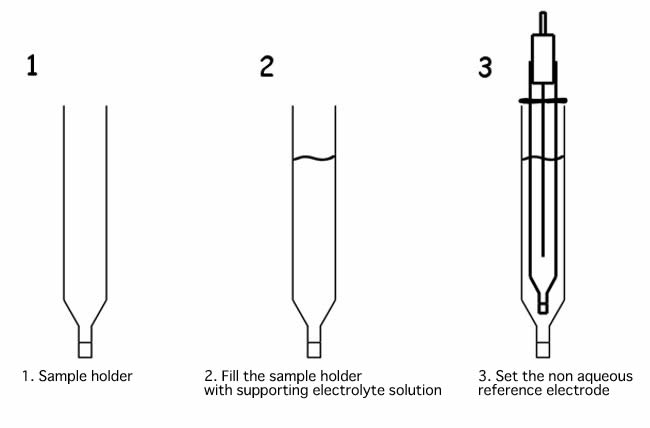
If you want to use the reference electrode in such way, use the SVC-2 Voltammetry cell. And, when a new sample holder will be used, keep immersed in a solution overnight before use. Permeating the solution to the porous of IPPG, increases the permeability of the ion through them, making able to carry out the cyclic voltammetry experiments.

