TOP > Technical note > Basics for who are starting electrochemistry > Reference electrode
This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
The use of a reference electrode necessarily introduces a liquid-liquid interface between the reference electrode and a test solution. The use of a salt bridge containing a reference electrode adds further an extra interface. A junction potential inevitably appears at such the interface depending on the composition of electrolyte solutions. The different ionic mobility of constituent ions through the interface is the origin of a junction potential. The magnitude of junction potential is calculated using ionic mobility and concentration of constituent electrolyte according to Henderson’s equation. Although the equation looks complicated with involvement of ionic mobility, ionic charge and concentration of constituent ions, it is simplified since only a few major components are effective.
A basis to make junction potential as small as possible is to choose anion and cation in similar mobility and in higher content as possible. For example, as ionic mobility of K+ and Cl- is similar, while that of Na+ is about 0.7 of K+, a choice of KCl is better than that of NaCl. As H+ and Cl- are of remarkably different mobility (H+ moves about five times faster than Cl-), a use of HCl is disadvantageous. When you cannot help using HCl, you can escape by using higher concentration of KCl than HCl. For example, the junction potential between 1 M NaCl and 1M KCl solution is 4.3 mV, while one between 1 M NaCl and 3 M KCl solution is only 1.7 mV. That between 1 M HCl and 1 M KCl solution is up to 27 mV, while that between 1 M HCl and 3 M KCl solution is down to 16 mV.
When you use a salt bridge between reference electrode and test solution, it is important to make junction potential as less as possible by a judicious choice of electrolyte component. The combination of K+, NH4+, Cl-, NO3- is often chose because of similar mobility of them.
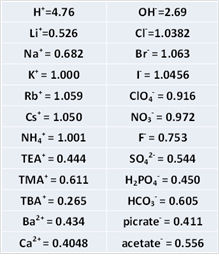
As the solubility of KCl is rather low (about 4.8 M at saturated in water), CsCl or RbCl is sometimes employed at the expense of cost. The mobility of Rb+ and Cs+ is almost identical to Cl-. The relative mobility of other ions as KCl = 1.000 is shown in right Table.
Sometimes NaCl is employed instead of KCl, when perchlorate anion is involved in test solution. The reason is that the solubility of potassium perchlorate is rather low, while that of sodium perchlorate is about a hundred times higher than of potassium salt. The risk of deposition in the frit due to the low solubility of KClO4 is diminished by this precaution. Which one is employed might depend on a compromise among practical convenience.
The sample holder, which has vycor frit at the end of glass tube is available by BAS Japan. It is also usable for a salt bridge or a reference electrode.
This is a basic content about the types of reference electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
7. Junction potential
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe
When you use a salt bridge between reference electrode and test solution, it is important to make junction potential as less as possible by a judicious choice of electrolyte component. The combination of K+, NH4+, Cl-, NO3- is often chose because of similar mobility of them.
As the solubility of KCl is rather low (about 4.8 M at saturated in water), CsCl or RbCl is sometimes employed at the expense of cost. The mobility of Rb+ and Cs+ is almost identical to Cl-. The relative mobility of other ions as KCl = 1.000 is shown in right Table.

The sample holder, which has vycor frit at the end of glass tube is available by BAS Japan. It is also usable for a salt bridge or a reference electrode.

