Working electrode selection
Choice of working electrode is a vital component for experiment.
Redox potential of molecule under research have to fall in the potential
window over which the background current due to the electrode itself
must not disturb the experiment.
Pt (Platinum) and Au (Gold) electrodes - applied to organic or inorganic
substances measurement because they have high overpotential for
oxygen evolution and low overpotential for hydrogen evolution.
Hg (Mercury) electrode - well suited to measure reduction of metal ions
because of its high overpotential for hydrogen evolution. Hg electrode
can easily observe the reduction of Zn.
At the measurement with non-aqueous solvent, hydrogen/oxygen overpotential does not affect. Instead of that, decomposition potential of the
non-aqueous solvent and supporting electrolyte comes to an important
factor. Furthermore, impurity of water in non-aqueous solvent makes the
potential window narrower depending on the quantity of the water.
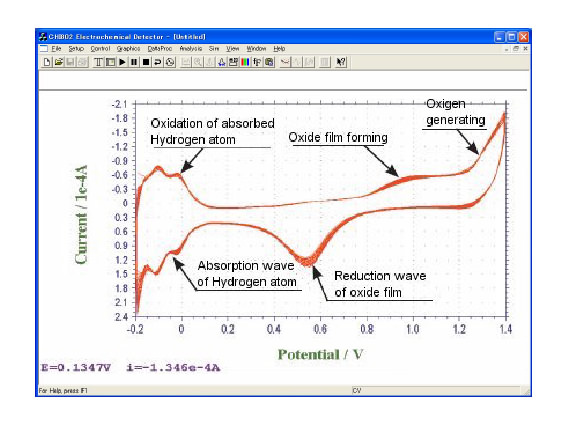
Figure: Current-Potential curbs of the result of 50 times' scanning by immersing a Pt electrode in 0.5 M H2SO4 solution. Reiteration of the cycle obviously activates the electrode. Although there is absorption wave at the cathode side, it is possible to observe other electrodes' reaction in this potential range because this reaction requires only certain quantity of electricity.
Working Electrode - More Information
![]()

