3.Rotating disk electrode (RDE) measurement
3.1. Feature and measurement of RDE instrument
- [Feature of RDE instrument]
-
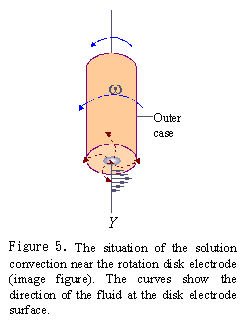
When RDE electrode is rotated in the solution, the spinning disk drags the fluid at its surface and, because of centrifugal force, flings the solution outwards from the center in a radial direction. The fluid at the disk surface is replenished by a flow normal to the surface. The convection occurs near the disk surface.
Because there is a thin layer rotating together with the disk electrode, the convection velocity of solution from the disk surface to distance Y is distributed as shown in Fig.5. The convection velocity of the solution is improved with the increasing of the rotation rate, as a result, the diffusion layer becomes thin and diffusion rate is increased. It means that the diffusion rate of substrate is regulated by controlling the electrode rotation rate.
For normal static electrode measurement, such as cyclic voltammetry, in order to analyze mass transfer process and electron transfer process, the potential scan rate is changed. The peak current difference can be found under different scan rates.
One feature of the RDE is the smallest concentration variation of the substrate near the electrode surface, so the current does not depend on time and shows a constant value. In the case of RDE measurement, under some potential scan rate (dependent on systems studied, usually, tens of mV/s), the diffusion limiting current is independent of scan rate. Hysteresis between positive scan and negative scan almost can not be found. The current-potential curve obtained using RDE is called hydrodynamic voltammogram.
- [Apparatus used for RDE Measurement]
- The instruments required for RDE measurement are listed below:
- a) Potentiostat (e.g. ALS600C or 700C electrochemical analyzer)
b) Personal computer for potentiostat control (generally Windows PC)
c) counter electrode (Pt winding wire)
d) reference electrode (suitable type for the electrolyte)
e) rotating disk working electrode (e.g. ALS RDE, disk electrode portion of RRDE electrode)
f) cell for RDE
g) electrode rotator (e.g. ALS RRDE-3A)
a)∼d) are the same as what are used in the usual electrochemistry measurement, such as cyclic voltammetry. e)∼g) are special equipments for RDE measurement.
3.2. Experiment for fast electron transfer redox pair [Fe(CN)63−/Fe(CN)64−]
The redox reaction with electrode surface electron transfer rate higher enough than the substance diffusion rate is called a reversible system.
- Fe(CN)63− + e− ⇔ Fe(CN)64- Eq. 1
[Fe(CN)63−/Fe(CN)64−] is a typical reversible system. In generally, a reversible system for RDE measurement can help to obtain the diffusion coefficient D of substrate, reversible half peak potential E1/2 and electron transfer number n.
- [Electrolyte Preparation]
- 2 mM K3[Fe(CN)6] including 0.1 M KNO3 was prepared for the measurement. High purity reagents were dissolved in pure water (nano-purity or distilled water). The concentration of substrate K3FeCN6 should be prepared exactly. Please pre-electrolyze the supporting electrolyte solution beforehand using the method reported in literature4 if the measurement time is long.
- [Setup for disk electrode]
- a) Electrode surface was polished with alumina and rinsed with pure water before use.
b) The electrode was set to the shaft.
c) Turned shaft with hand and checked whether the electrode surface was rotated horizontally without eccentricity.
d) The rotation number was changed from 500 rpm to 5000 rpm to confirm whether there was any shaking or abnormal noise.
- [RDE cell]
- a) Glass cell and Pt counter electrode were washed with water and rinsed with pure water. After rinsing with a small amount of electrolyte solution, the electrolyte solution was poured to cell up to predetermined level.
b) The reference electrode (Fig.6) beforehand rinsed with pure water was set up.
c) Working electrode was dipped into the solution and adjusted to the proper height and center position of cell cover, so that the turbulent flow might not occur.
d) Nitrogen gas was used for purge (about 20 ∼ 30 minutes) to remove dissolved oxygen (If you use RRDE-3A, manual and remote purge time control are available). Please remember to adjust the nitrogen gas to minimum flow rate before measurement.
- [Connection and setup for electrode rotator and potentiostat]
- All leads of electrode rotator and potentiostat including the leads to reference electrode (Ag/AgCl) and counter electrode were connected. After finishing above preparation, make sure no air bubbles in disk electrode surface and reference electrode liquid-liquid junction part.
- [CV measurement with RDE electrode]
- After setting the instrument, the power source turns on and starts the ALS software window of PC. CV measurements with scan rate changing in 100, 50, 25,10 mV/s and CV plots are recorded simultaneously.
- [CV judgment]
- CV curves are shown in Fig.7. The feature for reversible system describes in below:
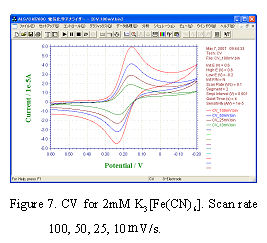
a) Potential for peak current is independent of scan rate.
b) The difference of anode peak potential and cathode peak potential is 59 mV/n (n is the number of reaction electron) at 25°C.
c) Peak current is proportional to v1/2.
CV curves of Fig.7 fulfill above conditions mostly, and the RDE electrode surface is considered to be normal. If the electrode is found to be dirty , electrochemical cleaning is required. If the dirty can not be still removed, please exchange the solution and examine the same operation. If the problem is still there, don’t be hurry. It is better to re-polish the electrode and re-prepare the solution.
- [Measurement for Hydrodynamic Voltammetry]
- a) Scan rate was set to 10 mV/s and segment was set to 1, the parameters were same to CV.
b) The electrode rotated at e.g. 500 rpm and rotation stability was checked.
c) The potential scans to negative direction(0.6 V ∼ -0.2 V) and data record in the same procedure as CV measurement. The rotation rates change (e.g. 500, 1000, 2000, 3000, 4000, 5000 rpm) and corresponding current-potential voltammograms record.
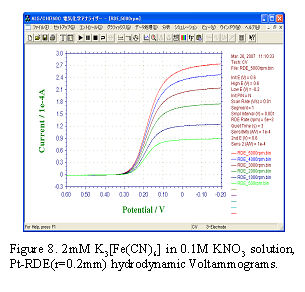
- [Data analysis – Levich plot]
- Fig.8 shows hydrodynamic voltammograms. If cathodic polarization is enough (in this case E < 0 V), diffusion limited current (or limiting current) iL can be observed.
iL is proportional to square root of electrode angular rotation rate (ω1/2) as shown in Eq.2.
- iL = 0.62 n F A D 2/3 ω1/2 ν-1/6 C∗ Eq.2
- F is faraday constant 96,485 (C/mol), A is the electrode surface area (cm2/s), ω is the electrode angular rotation rate(rad/s), v is the kinetic viscosity (cm2/s) of solution, C∗ is the bulk concentration of substrate (mol/cm3). The relationship for angular rotation rate ω to rotation rate f (rpm) is ω = 2πf/60.
iL currents obtained under various rotating rate were plotted versus ω1/2 and a straight line (Levich plot) which pass though the zero point was obtained in Fig.9. The diffusion coefficient D, the value of reaction electron n, etc. can be calculated from the slope. At 25°C pure water value of 0.01 cm2/s was used as kinetic viscosity of solution, and number of reaction electron n = 1, C = 2 x 10-6 mol/cm3, A = 0.1256 cm2 were used for equation (5). The diffusion coefficient D = 6.73 x 10-6 (cm2/s) of [Fe(CN)6]3- could be obtained from the slope. If the electrochemical reaction is accompanied by preceding or the succeeding chemical reaction, the plot will deviate from the straight line.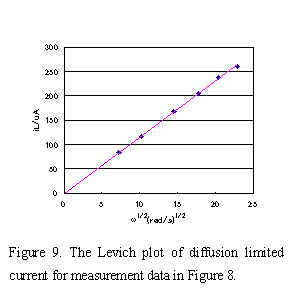
3.3. Experiment for slow electron transfer system (oxygen reduction)
The slow electron transfer process on the electrode surface is called irreversible system (It does not mean the reverse reaction not occurring). An example of the typical reaction is the reduction of dissolved oxygen in acidic solution.
- O2 + 2e− + 2H+ → H2O Eq.3
- O2 + 4e− + 4H+ → 2H2O Eq.4
The information obtained from RDE measurement for irreversible system are kinetically-controlled current and the catalyst electron transfer number Napp, which can be calculated from the slope of a Koutecky-Levich plot in the case of electrode catalyst.
- i-1 = ik-1 + (0.62 n F A D2/3ω1/2v-1/6C∗)-1 Eq.5
Eq.5 is called Koutecky-Levich equation. The first term on the right side of equation is activity determining current which is not dependent on rotation rate. The second term is a reciprocal of Eq.2 and means the mass transfer resistance depending on rotation rate.
- [RDE measurement for reduction of oxygen]
- Pt working electrode (disk part of Pt-Pt RRDE electrode polishes with alumina and cleaned before use) sets to RRDE-3A, and Ag/AgCl reference electrode and Pt winding wire counter electrode put into the cell. The electrolytes used were 0.2 M Na2SO4 and 0.1 M H2SO4, the saturated oxygen solution was obtained by purging the oxygen gas for 30 minutes. At first, the potential was scanned on the electrode in stationary state and the proper potential scan range (0.8 V ∼ -0.2 V) was determined. Next, the RDE measurement was carried out at scan rate 25 mV/s while the electrode was rotating and the voltammogram was recorded simultaneously (Fig.10).
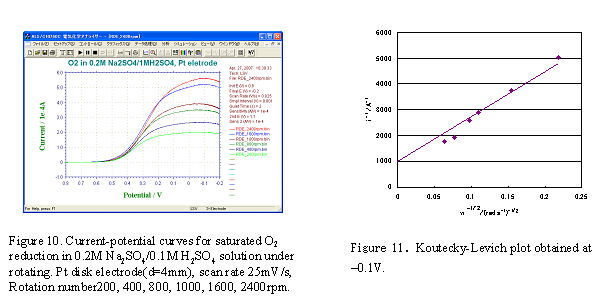
The currents corresponding to a fixed potential (-0.1 V) for various rotation rates in Fig.10 were plotted vs. ω-1/2, a straight line was obtained in Fig.11. Using the dissolved oxygen concentration (C∗ = 1.27 × 106 mol/cm2), O2 diffusion coefficient (Do = 2.0 × 10-5 cm2/s), solution kinetic viscosity (v =0.01 cm2/s), electrode surface area (A = 0.125 cm2), and faraday constant (F = 96,485 C/mol), the reaction electron number n = 3.6 (4 electrons reaction) can be obtained from the slope value.
References
4) M. Watanabe, Denki kagaku (presently Electrochemistry),53, 671 (1985).
5) C.Paliteiro, A. Hamnett and J.B. Goodenough, J. Electroanal. Chem., 233, 147 (1987).
Technical note
Hydrodynamic Voltammetry Measurement Using
Rotating Disk Electrode (RDE) and Rotating Ring Disk Electrode (RRDE)
- Introduction
- Rotating disk electrode (RDE) measurements
- Rotating ring disk electrode (RRDE) measurement

