Part 6: Redox Potential
This is a basic introduction to the redox potential in electrochemistry.
The topics are listed below:
- Redox Potential I: About HOMO and LUMO
- Redox Potential II: About Ferrocene
- Redox Potential III: Factors that determine redox potential
- Redox Potential IV: Organic transition metal complex
- Redox Potential V: Linear relationship between HOMO energy and redox potential
Redox Potential III: Factors that determine redox potential
Professor Noriyuki Watanabe
Regarding many organic transition metal compounds, when they are coordinated by the carbonyl (CO) which is a π electron acceptor, the redox potential may have a shift to the positive direction in large extent. This is due to the electrons in π symmetric orbitals of the central metal are reversely supplied (back donation) to the anti-particulate π orbits of the ligand, resulting the electron density of the central metal decreasing. Metal carbonyl compounds generally have high potentials and their potentials increase proportionally to the number of the carbonyl groups (Fig. 8-1).
Consequently, it is possible to estimate the extent of reverse donation according to infrared spectrums, and the redox potential measurements are often used in combination with IR measurements. An example for the metal cluster8-1) in Fig.8-2 is shown below.
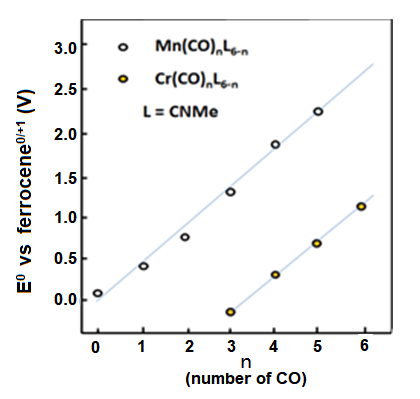 Fig. 8-1 The redox potential change of the manganese and chromium compounds when carbonyl groups substitute methyl isocyanate
Fig. 8-1 The redox potential change of the manganese and chromium compounds when carbonyl groups substitute methyl isocyanate
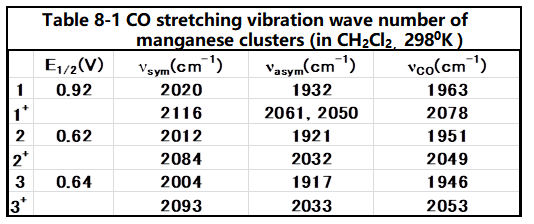
The reversible redox potential of cyclopentadienyl manganese tricarbonyl (1) is 0.92 V vs. ferrocene reference.
The IR spectrum in the carbonyl region during the bulk electrolytic oxidation is shown in Fig.8-3.
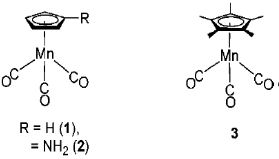
Fig.8-2 Manganese clusters
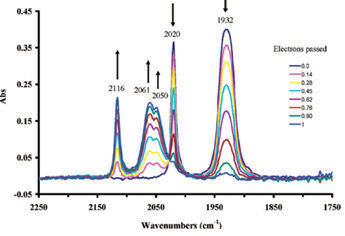
Fig. 8-3 Infrared spectrum changes of compound 1 during bulk electrolytic Oxidation
With electrolytic oxidation proceeding, the absorption intensity of two peaks belonging to 1 at the low wavenumber side (1930 cm-1, 2020 cm-1) are decreased, meanwhile three peaks (2050 cm-1, 2061 cm-1, 2116 cm-1) belonging to 1+ appear at the high wave number side, and their absorption intensity increase gradually.
Electrolytic oxidation causes the electron intensity decreasing around the manganese, thus the electron back donation is decreased. Therefore, the Mn-CO bond is weakened; on country indicate the enhancement of the CO bond.
The redox potentials of compounds 2 and 3 (Fig. 8-2) with amino or methyl substitutions on cyclopentenyl rings are in the negative direction comparing with compound 1.
This is due to the electron supplied property of the substituents, which increases the electron back donation from the central metal to the ligand CO, and the wavenumber of CO shifts to be low, in the IR spectrum of the CO stretching frequency (see below table).
Reference:
8-1) W.E. Geiger et al., J. Am. Chem. Soc., 130, 9859 (2008)

