Part 6: Redox Potential
This is a basic introduction to the redox potential in electrochemistry.
The topics are listed below:
- Redox Potential I: About HOMO and LUMO
- Redox Potential II: About Ferrocene
- Redox Potential III: Factors that determine redox potential
- Redox Potential IV: Organic transition metal complex
- Redox Potential V: Linear relationship between HOMO energy and redox potential
Redox Potential V: Linear relationship between HOMO energy and redox potential
Professor Noriyuki Watanabe
Mn[(CO)6-n(CNR)n]+, mentioned in the Redox Potential - III, is a representative example of the linear relationship between the HOMO energy and the redox potential of an organometallic compound by molecular orbital calculations10-1). The carbonyl is an σ-donor and a strong π-acceptor. Alkyl isocyanate is also an σ-donor and π-acceptor, but its π-acceptance is not as strong as that of carbonyl. As mentioned previously, when CO is bonded, t2g electrons from the metal d-orbital (also π-active) interact strongly with the π∗ orbital of CO (the semi-bondable orbital of CO), resulting in a reverse donor from the metal to the carbonyl. In the previous article, we stated that the redox potential is tilted in the positive direction because the t2g orbital is pushed down by the interaction with the π∗ orbital. The density of electrons around the metal is reduced due to the donation of electrons to the ligand. As a result, the redox potential is shifted in a positive direction because the metal center is less likely to be oxidized. Alternatively, the electron density around the metal is reduced and the effective nuclear charge is increased. The reduction of the HOMO level leads to a positive shift in the redox potential. In any case, the redox potential shifts positively when the number of COs to be bounded increases. Conversely, the reduction of the number of COs (and increase of n) shifts the redox potential in a negative direction.
Pletcher et al.10-1) gave the following equation for this relationship.
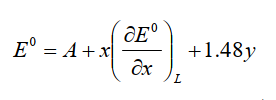
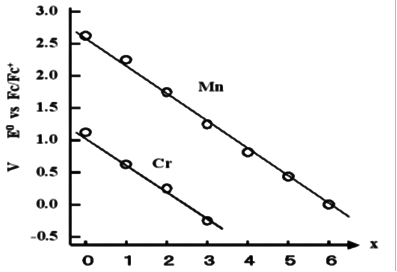
Fig.10-1. Number of acceptors and redox potential.
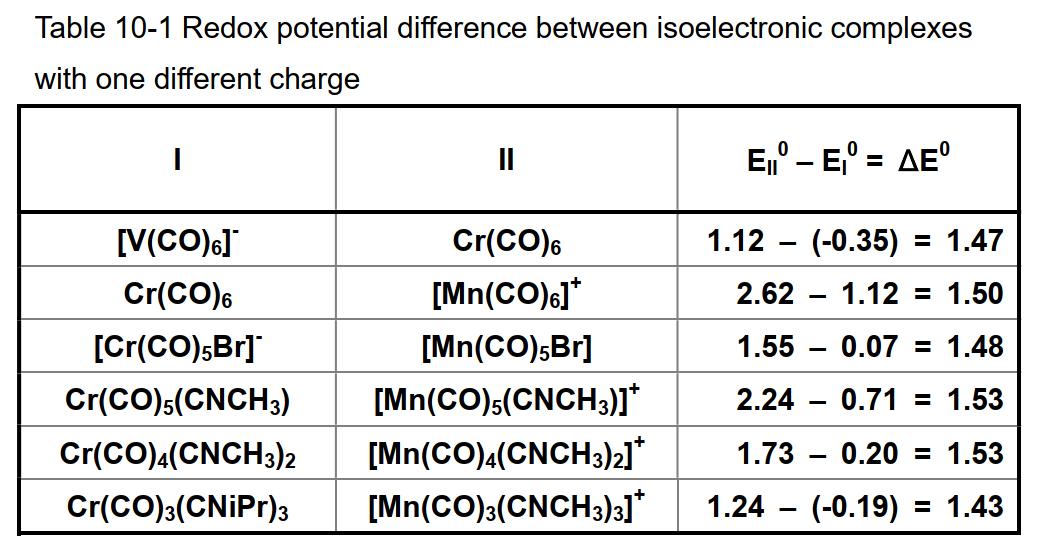
A is a constant determined by the medium (solvent and supporting electrolyte) used and the reference electrode, x is the number of ligands to substitute the carbonyl, and y is the charge of the complex. The coefficient of 1.48 for y is calculated from the average of the measured values of the carbonyl complex with the same number of carbonyls and the isoelectronic structure (table below). If the charge is increased by one, it means a shift of 1.48 V in the positive direction. This value is generally informative.
In redox systems that are similar on the periodic table with adjacent atoms, generally a metals with small atomic numbers tends to show redox reactions to more negative potential. This is true for the V, Cr and Mn series of hexacarbonyl isoelectric complexes. [ V-(CO)6; -0.35V, Cr(CO)6 ; +1.12V, Mn+(CO)6 ; +2.62V ]
Reference:
10-1) Pickett, Pletcher, J. Organometal. Chem., 102, 327 (1975)

