TOP > Technical note > Basics for who are starting electrochemistry > Basics and applications of electrochemistry > CV (Cyclic Voltammetry > CV (Cyclic Voltammetry) - (1)
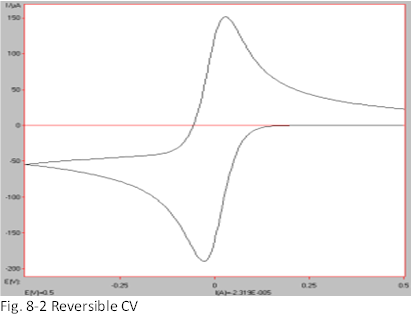
Fig. 8-2 shows a typical CV of a reversible system for a redox reaction with electron transfer, O + e ⇄ R. In a reversible system with one electron transfer, the width of the peak potential between oxidation and reduction is almost 60 mV. If the system is not reversible, the potential width will be much wider. This will be discussed in a later section. As the oxidized O is reduced to the reduced R, concentration gradients appear at the electrode surface for both O and R, and diffusion occurs along these gradients (Diffusion is the movement from denser to thinner, so the oxide O consumed at the electrode surface moves from the offshore to the electrode direction, while the generated R moves from the electrode surface to the offshore).
As previously described, the current value is proportional to the concentration gradient of the active material at the electrode surface, and the changes in the concentrations of O and R as the CV progresses are plotted against the distance from the electrode surface in Fig. 8-3 and 8-4, the left edge of the light green background is the electrode surface and the direction of solution off-set is toward the right. The vertical axis is the concentration. The bulk concentration of the active material is 1 mM. The figure shows how the active material concentration (black line) and product concentration (red line) change from the electrode to the offshore of the solution.
The current is proportional to the concentration gradient and diffusion coefficient at the electrode interface (i∝D(∂C/∂x)x=0), and how the concentration of the active material on the electrode surface changes with potential sweep. Awareness helps to understand the reactions and CV profiles occurring at the electrode interface. Near the rise of the peak in Fig. 8-3 (near the red circle on the far right of the CV), the electron transfer rate is still slow, and the substance is sufficiently supplied. This is the called electron transfer rate determining state.
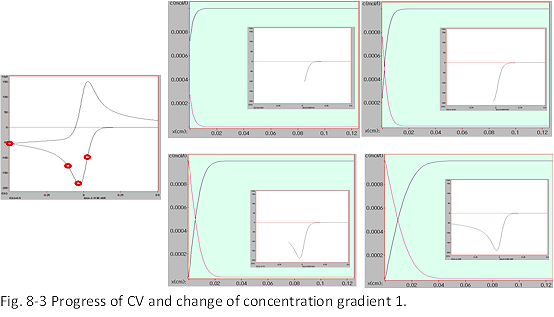
As the potential becomes deeper, the electron transfer rate increases rapidly, and the supply due to diffusion and the disappearance on the electrode surface are in a balanced state, and the current peak (second red circle from the right, the surface concentration gradient is the maximum). After the peak, the electron transfer rate is sufficiently high, the supply cannot catch up, and the current decreases (near the red circle, which is the third from the right).
At this point, the concentration of active material O on the electrode surface The concentration profiles of the products shown by the red lines are zero (because the current is proportional to the concentration gradient), and the surface concentration gradient gradually decreases. At the same time, the position of half the concentration extends offshore. The concentration profiles of the products shown by the red lines are almost symmetrically folded versions of the concentration profiles of the oxides (perfectly symmetric if the diffusion coefficients of O and R are the same).
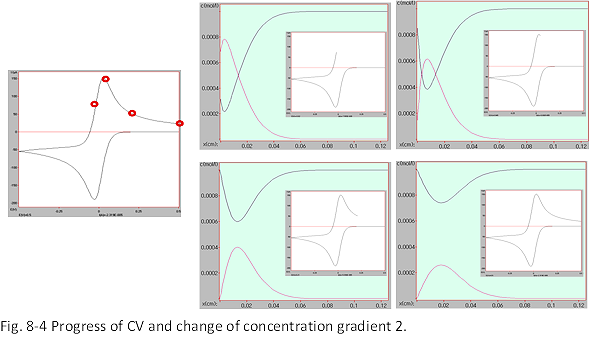
When the potential sweep is turned back (reverse sweep, Fig. 8-4), the oxidation reaction of the reduced product, which is a product near the electrode, starts before the current zero line is crossed. A peak can be seen in the product concentration profile. By turning it over, the active material is regenerated and the surface concentration returns to the original level. The minimum and maximum appear in the concentration profiles of O and R, respectively. In the vicinity of the minimum and maximum, the direction of movement is a mixture of the electrode surface direction and the offshore direction, resulting in a complicated movement, but the peak position gradually extends offshore.
Part 8: CV (Cyclic Voltammetry)
This is a basic introduction to the cyclic voltammetry.
The topics are listed below:
- CV (Cyclic Voltammetry): Part 1
- CV (Cyclic Voltammetry): Part 2
- CV (Cyclic Voltammetry): Part 3
CV (Cyclic Voltammetry): Part 2
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe

As previously described, the current value is proportional to the concentration gradient of the active material at the electrode surface, and the changes in the concentrations of O and R as the CV progresses are plotted against the distance from the electrode surface in Fig. 8-3 and 8-4, the left edge of the light green background is the electrode surface and the direction of solution off-set is toward the right. The vertical axis is the concentration. The bulk concentration of the active material is 1 mM. The figure shows how the active material concentration (black line) and product concentration (red line) change from the electrode to the offshore of the solution.

As the potential becomes deeper, the electron transfer rate increases rapidly, and the supply due to diffusion and the disappearance on the electrode surface are in a balanced state, and the current peak (second red circle from the right, the surface concentration gradient is the maximum). After the peak, the electron transfer rate is sufficiently high, the supply cannot catch up, and the current decreases (near the red circle, which is the third from the right).

When the potential sweep is turned back (reverse sweep, Fig. 8-4), the oxidation reaction of the reduced product, which is a product near the electrode, starts before the current zero line is crossed. A peak can be seen in the product concentration profile. By turning it over, the active material is regenerated and the surface concentration returns to the original level. The minimum and maximum appear in the concentration profiles of O and R, respectively. In the vicinity of the minimum and maximum, the direction of movement is a mixture of the electrode surface direction and the offshore direction, resulting in a complicated movement, but the peak position gradually extends offshore.

