TOP > Technical note > Basics for who are starting electrochemistry > Basics and applications of electrochemistry > CV (Cyclic Voltammetry > CV (Cyclic Voltammetry) - (1)
This can be understood by considering the concentration distribution of the products (Fig. 8-5). The right side of Fig. 8-5 shows the situation immediately after the first sweep. The concentration peak of the reduction products is located near the electrode (almost at the same position as the concentration). Since the concentration is higher than the electrode surface, it diffuses toward the electrode surface. This is due to the oxidation current (note that the oxide moves away from the electrode in the opposite direction).
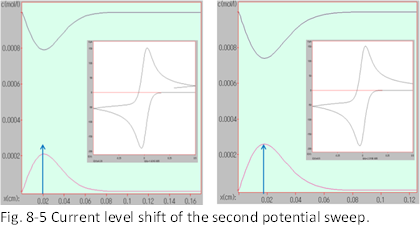
The concentration profile at a certain point after the start of the second sweep is shown in the left panel of Fig. 8-5. The peak concentration of the product clearly decreases. It may be difficult to notice, but the peak position is farther away from the electrode in the left figure.
Note that the distance scale from the electrode is drawn slightly smaller in the left figure (due to the passage of time). You can see that the peak concentration in the left figure is clearly decreasing. This is because it is consumed by the oxidation reaction. It will be helpful to understand the CV profile if you can picture the extinction of the reactive species near the electrode.
In addition to mentioning the proportional relationship between the concentration gradient on the electrode surface and the current value, I would like to make a few additional remarks that may seem persistent.
When using a rotating electrode or a microelectrode, a flat current response as shown in Fig. 8-6 can be obtained when sweeping the potential (Fig. 8-6-4). This is a saturated current response with no decay due to diffusion. The active material is sufficiently supplied by forced convection in the rotating electrode and by spherical diffusion in the microelectrode, Both by efficient transport.
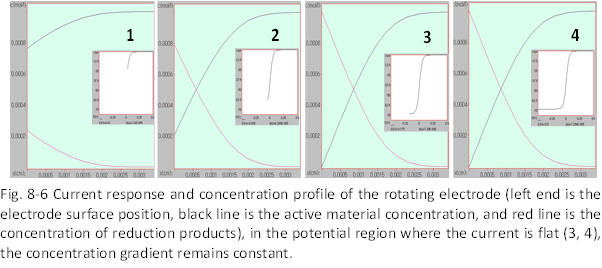
Thus, please note that the concentration gradient at the electrode surface at this time remains constant during the potential sweep.
When the electrode reaction product changes itself (e.g. decomposition) or reacts with coexisting materials in solution, the interfacial concentration is affected and the waveform deviates significantly from the reversible system. Chemically, this is called an irreversible system. The above examples are all the simplest, chemically and electrochemically reversible cases.
Part 8: CV (Cyclic Voltammetry)
This is a basic introduction to the cyclic voltammetry.
The topics are listed below:
- CV (Cyclic Voltammetry): Part 1
- CV (Cyclic Voltammetry): Part 2
- CV (Cyclic Voltammetry): Part 3
CV (Cyclic Voltammetry): Part 3
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe
Have you ever wondered why there is a shift in the current level during the second cycle of the potential sweep?

The concentration profile at a certain point after the start of the second sweep is shown in the left panel of Fig. 8-5. The peak concentration of the product clearly decreases. It may be difficult to notice, but the peak position is farther away from the electrode in the left figure.
Note that the distance scale from the electrode is drawn slightly smaller in the left figure (due to the passage of time). You can see that the peak concentration in the left figure is clearly decreasing. This is because it is consumed by the oxidation reaction. It will be helpful to understand the CV profile if you can picture the extinction of the reactive species near the electrode.
In addition to mentioning the proportional relationship between the concentration gradient on the electrode surface and the current value, I would like to make a few additional remarks that may seem persistent.

When the electrode reaction product changes itself (e.g. decomposition) or reacts with coexisting materials in solution, the interfacial concentration is affected and the waveform deviates significantly from the reversible system. Chemically, this is called an irreversible system. The above examples are all the simplest, chemically and electrochemically reversible cases.

