This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
2. Polarizable electrode and nonpolarizable electrode
Professor Noriyuki Watanabe
Electrodes can be divided into two types: polarizable and non-polarizable electrodes.
The characteristic of an ideal polarizable electrode is that no faraday current flow when the electrode potential is varied. This type of electrodes usually can be used as Working or Counter electrodes.
The feature of a non-polarizable electrode is once the electrode potential be changed, the Faraday current flows out. In generally, this type of electrodes can be used as Reference electrodes.
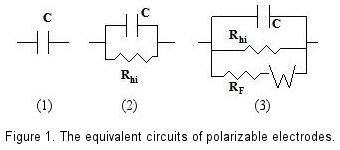
An ideal polarizable electrode can be represented by a capacitor (condenser) in equivalent circuit as shown in Fig. 1-1. However, the extremely weak current flows at an actual polarizable electrode indeed. Therefore a high value resistance (Rhi) is required to parallel with the capacitor to represent the actual polarizable electrode in equivalent circuit [Fig. 1-(2)].
If a redox species coexists with the polarizable electrode, the redox reaction of species is occurred on the electrode surface and the Faraday current flows under the certain potential. In this case, a potential depending variable resistance (RF) should be added to the equivalent circuit in parallel. Furthermore, the effect of the species diffusion should be included, and a Warburg Impedance element is connected to Faraday resistance (RF) in equivalent circuit [Fig. 1-(3)].
Figure 1 is a schematic view of above description. In the absence of a redox system, even electrode potential has been changed, the capacitance and high resistance parallel circuit could still be maintained, this variable potential range is so called as potential window.
The wide potential window of the polarized electrodes is a very important condition in practical application. Platinum, Gold and Carbon (e.g., glassy carbon etc.) electrodes can meet this condition in general.
Gold, Platinum and other metal electrodes
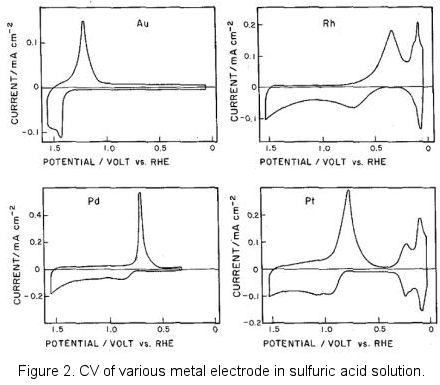
This redox paired phenomenon can be founded in most of the solid metal electrode.
When the concentration of the target component is very low, such background current will interfere the measurement and the potential window becomes narrow. Although the electrode potential window is theoretically regulated by the electrode capacitance potential range, there should be no influence for the application if the background current does not interfere to the measurement.
Incidentally, figure 2 in above was borrowed from the old literature, negative potential located at the potential axis right side which was known as the classic graphic display method. This reflected the epoch in which the polarography was popularly used in the metallic ions reduction. Currently, the opposite direction graphic display of IUPAC is mainly used (positive right).

