TOP > Technical note > Basics for who are starting electrochemistry > Working electrode
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
In previous technical note, it was mentioned that GC electrode activity could be improved by activated carbon depredated organic solvent cleaning, also the pyridine cleaning could achieve to same result. At pyridine cleaned GC electrode, not only the electron transfer rate of 4-methylcatechol increases but also its adsorption on electrode increases significantly.
As shown in Fig. 8-1, the cyclic voltammogram of 10 µM 4-methylcatechol (pH=1) micro sample has a major feature of diffusive wave on polished only GC electrode, in comparison, on the further pyridine cleaned GC electrode, CV has a major adsorbent wave with a minor diffusive wave in features, the decrease of redox peak potential difference range could be noticed. The catechol adsorption on GC electrode plays an important role in electron transfer, is the theme of this issue. The below two experimental results may support this opinion.
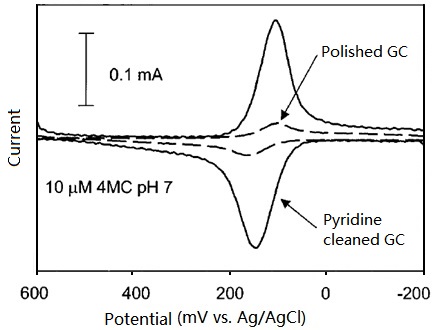
Fig. 8-1 Cyclic voltammograms of 1 µM 4-methylcatechol (pH=1) at polished only and further pyridine cleaned GC electrodes respectively.
One is that on the GC electrode with trifluoromethylphenyl (TFMP) monomolecular film modification, the electron transfer rate of catechol dwindles significantly (the peak potential width becomes quite wider than 60 mV of general one-electron reversible system), till the electron transfer becomes unobserved at all. This feature is obviously different from other outer-sphere electron transfer molecules (outer-sphere electron transfer molecules are hardly affected by the electrode surface state. Even if they are affected, the change in current is about 2 to 10 times at mostly, but in the case of catechol, the tremendous change is in digits, for example 1000 times.).
For example, on the trifluoromethylphenyl group (TFMP) coated GC electrode, the electron transfer of dopamine (DA) abruptly slows corresponding to TFMP coating ratio increase (It may be found from the significant increase of the peak potential width in Fig. 8-2).
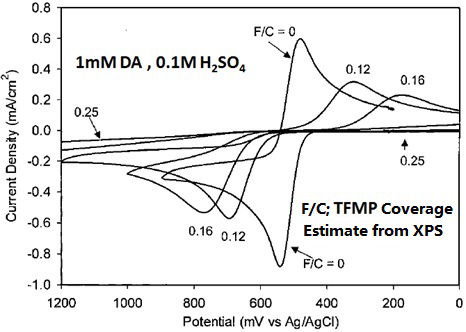
Fig. 8-2 Cyclic voltammograms of 1 mM dopamine in 0.1 M H2SO4 solution on GC electrodes with various TFMP coating ratio (0.12, 0.16, 0.25).
Such an obvious decrease of electron transfer rate may also be achieved by the adsorption of mono-molecules such as methylene blue.
However, in the case of catechol derivative molecules such as quinone adsorption, the electron transfer rate of dopamine and 4-methylcatechol just decreases slightly, although their adsorption is disturbed (Fig. 8-3). This is the second reason.
On catechol analogue duroquinone monomolecular adsorbed electrode, the peak potential width of dopamine (comparing with the activated carbon cleaned electrode) has a little increase. On the other hand, the methylene blue monomolecular adsorption is notablely different (Waves at potential range from 200 mV to negative are due to the adsorption of methylene blue and duroquinone in Fig. 8-3). Thus, the adsorption of quinone including adsorption of catechol itself plays a major role in electron transfer of catechol.
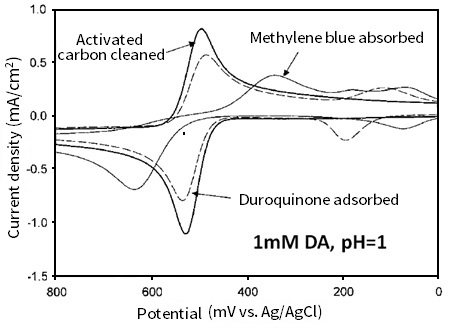
Fig. 8-3 Cyclic voltammograms of 1 mM dopamine (pH = 1) on glassy carbon electrodes with different surface treatments (activated carbon cleaned, duroquinone adsorbed and methylene blue adsorbed).
References:
8-1) S.H. DuVall and R.L. McCreery, Anal. Chem., 71, 4594, (1999)
8-2) S.H. DuVall and R.L. McCreery, J. Am. Chem. Soc.,122, 6759, (2000)
8-3) A. Rene and D,H. Evans, J. Phys. Chem. C, 116, 14454, (2012)
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
8. Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe
The electrochemistry regarding Quinone-hydroquinone including Catechol has been continuously studied for many years and it is an important theme spanning various related fields. Recently there is also an interesting report by D. H. Evans et al. 8-3). Perhaps that story might touch either, relating to carbon electrode surface treatment, this time the introduction subject is also selected from literatures of McCreery et al. (8-1 - 8-2).
As shown in Fig. 8-1, the cyclic voltammogram of 10 µM 4-methylcatechol (pH=1) micro sample has a major feature of diffusive wave on polished only GC electrode, in comparison, on the further pyridine cleaned GC electrode, CV has a major adsorbent wave with a minor diffusive wave in features, the decrease of redox peak potential difference range could be noticed. The catechol adsorption on GC electrode plays an important role in electron transfer, is the theme of this issue. The below two experimental results may support this opinion.

Fig. 8-1 Cyclic voltammograms of 1 µM 4-methylcatechol (pH=1) at polished only and further pyridine cleaned GC electrodes respectively.
For example, on the trifluoromethylphenyl group (TFMP) coated GC electrode, the electron transfer of dopamine (DA) abruptly slows corresponding to TFMP coating ratio increase (It may be found from the significant increase of the peak potential width in Fig. 8-2).

Fig. 8-2 Cyclic voltammograms of 1 mM dopamine in 0.1 M H2SO4 solution on GC electrodes with various TFMP coating ratio (0.12, 0.16, 0.25).
Such an obvious decrease of electron transfer rate may also be achieved by the adsorption of mono-molecules such as methylene blue.
On catechol analogue duroquinone monomolecular adsorbed electrode, the peak potential width of dopamine (comparing with the activated carbon cleaned electrode) has a little increase. On the other hand, the methylene blue monomolecular adsorption is notablely different (Waves at potential range from 200 mV to negative are due to the adsorption of methylene blue and duroquinone in Fig. 8-3). Thus, the adsorption of quinone including adsorption of catechol itself plays a major role in electron transfer of catechol.

Fig. 8-3 Cyclic voltammograms of 1 mM dopamine (pH = 1) on glassy carbon electrodes with different surface treatments (activated carbon cleaned, duroquinone adsorbed and methylene blue adsorbed).
References:
8-1) S.H. DuVall and R.L. McCreery, Anal. Chem., 71, 4594, (1999)
8-2) S.H. DuVall and R.L. McCreery, J. Am. Chem. Soc.,122, 6759, (2000)
8-3) A. Rene and D,H. Evans, J. Phys. Chem. C, 116, 14454, (2012)

