TOP > Technical note > Basics for who are starting electrochemistry > Working electrode
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
After normal polishing (by alumina fine powder dispersed in water), the state of GC surface is proposed as shown in Fig. 6-1.
The right side of Fig. 6-1 is the surface of the GC, and the left side is the GC bulk. Various functional groups containing oxygen atoms such as carbonyl, hydroxyl, carboxyl, catechol, p-quinone, lactone etc., are dispersed on the electrode surface.
On such kind of GC electrode surface, the influence for the redox reaction varies with the type of the molecular species. Oxygen amount evaluation by XPS, the O/C ratio is 10% to 14% immediately after polishing, and can be reduced to less than 2% by various surface treatments (vacuum heat treatment, polishing in cyclohexane, hydrogen plasma treatment, etc.
Although these processes themselves are practically meaningless, they are important in terms of understanding the properties of the electrode surface.
McCreery etc. are summarizing the procedures for evaluating GC electrodes as shown in Fig. 6-1.).
Fig. 6-1 Schematic representation of GC surface after polishing
Ru(NH3)63+/2+, IrCl63-/2-, Co(phen)33+/2+, Fe(phen)33+/2+, Co(en)33+/2+, Dopamine (D(DA), 4-methyl catechol (4-MC), Dihydroxyphenylacetic acid (DOPAC), Fe(CN)63-/4-, ferrocene, viologens, ascorbic acid (AA), Anthraquinone disulfate (AQDSD) etc., are used as test substances to evaluate and investigate the electrode characteristics.
These substrates have been used as redox indicators and the mediators for understating the redox potential of electrochemically active proteins (Ref. 6-1, 6-2).
The electron transfer of outer-sphere electron transfer type molecular species does not have much dependence to the electrode surface state (monolayer adsorption, presence or absence of oxygen functional group, etc.).
Ferro-ferricyanide is usually regarded as an outer-sphere type, but it seems not be capable for GC electrodes.
In fact, it should be noted that ferro-ferricyanide is actually very sensitive to the state of the electrode surface. On the other hand, the presence of adsorption sites formed by hydrogen bonds via OH groups is important for dopamine and NADH etc.
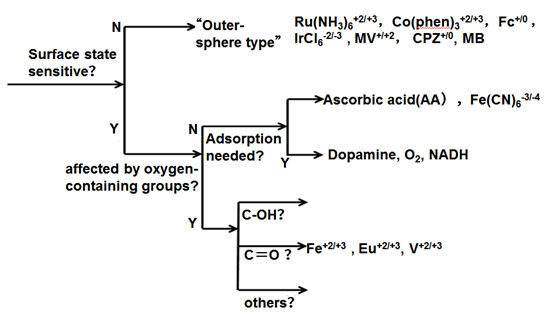
Fig. 6-2 Schematics of GC Surface evaluation procedures.
Besides, the hydrate ion of Fe2+/3+, Eu2+/3+ and V2+/3+ greatly depend on the presence or absence of a carbonyl group.
For example, the mono-layer molecular modification with dinitrophenyl hydrazine (DNPH) to crush the carbonyl group on the surface, the electron transfer of the outer-sphere electron transfer type Ru(NH3)62+/3+ is hardly affected (Fig. 6-3-c,d) but the electron transfer of Fe2+/3+ is changed to be extremely slow (Fig. 6-2-a). However, with adsorption of carbonyl group containing AQDS, the electron transfer of Fe2+/3+ is faster than non- adsorption on the GC electrode surface (Fig. 6-2-b) addition, the redox reaction of Fe2+/3+ is not much affected after the hydroxyl group crushing treatment with dinitrobenzyl chloride,due to the carbonyl groups remain (Ref. 6-3).
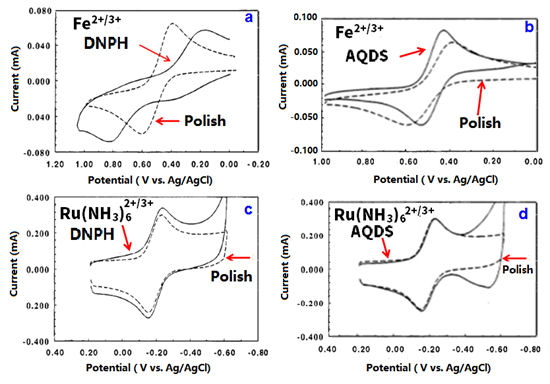
Fig. 6-3 Comparison of CVs of Fe2+/3+ and Ru(NH3)62+/3+ with polished surface and modified surface.
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
6. Glassy carbon surface state characteristics
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe
The right side of Fig. 6-1 is the surface of the GC, and the left side is the GC bulk. Various functional groups containing oxygen atoms such as carbonyl, hydroxyl, carboxyl, catechol, p-quinone, lactone etc., are dispersed on the electrode surface.
On such kind of GC electrode surface, the influence for the redox reaction varies with the type of the molecular species. Oxygen amount evaluation by XPS, the O/C ratio is 10% to 14% immediately after polishing, and can be reduced to less than 2% by various surface treatments (vacuum heat treatment, polishing in cyclohexane, hydrogen plasma treatment, etc.
Although these processes themselves are practically meaningless, they are important in terms of understanding the properties of the electrode surface.
McCreery etc. are summarizing the procedures for evaluating GC electrodes as shown in Fig. 6-1.).

Fig. 6-1 Schematic representation of GC surface after polishing
Ru(NH3)63+/2+, IrCl63-/2-, Co(phen)33+/2+, Fe(phen)33+/2+, Co(en)33+/2+, Dopamine (D(DA), 4-methyl catechol (4-MC), Dihydroxyphenylacetic acid (DOPAC), Fe(CN)63-/4-, ferrocene, viologens, ascorbic acid (AA), Anthraquinone disulfate (AQDSD) etc., are used as test substances to evaluate and investigate the electrode characteristics.
The electron transfer of outer-sphere electron transfer type molecular species does not have much dependence to the electrode surface state (monolayer adsorption, presence or absence of oxygen functional group, etc.).
Ferro-ferricyanide is usually regarded as an outer-sphere type, but it seems not be capable for GC electrodes.
In fact, it should be noted that ferro-ferricyanide is actually very sensitive to the state of the electrode surface. On the other hand, the presence of adsorption sites formed by hydrogen bonds via OH groups is important for dopamine and NADH etc.

Fig. 6-2 Schematics of GC Surface evaluation procedures.
Besides, the hydrate ion of Fe2+/3+, Eu2+/3+ and V2+/3+ greatly depend on the presence or absence of a carbonyl group.

Fig. 6-3 Comparison of CVs of Fe2+/3+ and Ru(NH3)62+/3+ with polished surface and modified surface.
References:
6-1) Electroanalytical Chemistry, 17, 221—374 , (1991).
6-2) R.L.McCreery, Chem. Rev., 108, 2646, (2008).
6-3) P.Chen, M.A.Fryling and R.L.McCreery, Anal. Chem., 67, 3115, (1995).

