TOP > Technical note > Basics for who are starting electrochemistry > Working electrode
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
McCreery et al. indicate that impurities contained in commercially available general organic solvent may possibly be adsorbed to the GC electrode and interfere with the electrochemical measurement. As a countermeasure, they recommend to purify the solvent before handling by using activated carbon, immersing the polished GC electrode in an activated carbon dispersed solvent for pre-cleaning. When GC electrode is immersed for a while in isopropanol with activated carbon dispersed, the impurities adsorbed to GC during polishing process are effectively removed by overwhelmingly large surface area of activated carbon adsorption.
An example for oxidation detection of 1 mM ascorbic acid in 0.1 M sulfuric acid by cyclic voltammetry (0.1 V/s) is shown in Fig. 7-1. Although the electron transfer reaction of ascorbic acid is fast, it is a typical example of EC reaction type and is chemically irreversible.
The solid line is for polished GC electrode, the dotted line is for isopropanol cleaned GC electrode, and the dashed line is for isopropanol containing activated carbon cleaned GC electrode, respectively in Fig. 7-1.
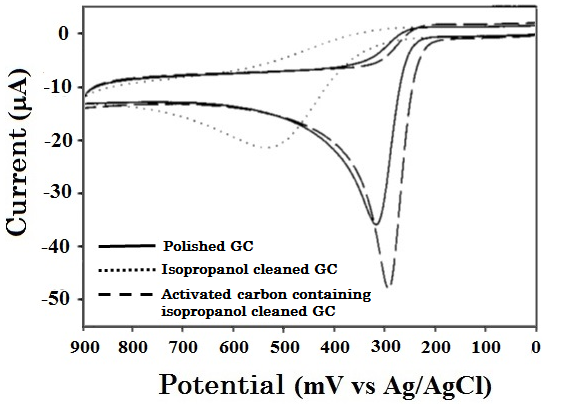
Fig. 7-1 Comparison of cyclic voltammograms of ascorbic acid measured by different cleaning treatment GC electrodes.
The oxidation peak potential of ascorbic acid at isopropanol cleaned GC electrode (Fig. 7-1, dotted line) shifts 200 mV to the positive direction comparing with the polished GC electrode (Fig. 7-1, solid line), indicating its electron transfer is slower than the polished GC electrode.
However, the oxidation peak potential of ascorbic acid using activated carbon depredated isopropanol solvent cleaned GC electrode (Fig. 7-1, dashed line) which shifts to the negative direction comparing with polished GC electrode (Fig. 7-1, solid line), indicating that electron transfer becomes faster than the polished electrode, because impurities adsorbed at the GC electrode surface are removed by adsorption to the activated carbon.
Let’s see another example (Fig. 7-2). Anthraquinone disulfate (AQDS) is easily adsorbed by the GC electrode surface, which is an electrode active material itself, it is a convenient substance when you want to investigate adsorption.
Cyclic voltammetry measurement for 40 µM AQDS in 0.1 M HClO4 using polished (solid line), acetonitrile cleaned (dotted line) and acetonitrile containing activated carbon cleaned (dashed line) GC electrodes are compared and shown in Fig. 7-2.
The CV profile exhibits a typical adsorption peak shape which has less attenuation from diffusion, and the small redox peak potential difference. In this case as well as in the previous example, comparing with the polished GC electrode, the peak area of just solvent cleaned GC electrode is decreased. Because the adsorption sites have already been occupied by the impurities in organic solvent, that AQDS can not be adsorbed on GC. Whereas, the peak area is doubled for the GC electrode which cleaned by acetonitrile containing activated carbon (Fig. 7-2, dashed line).
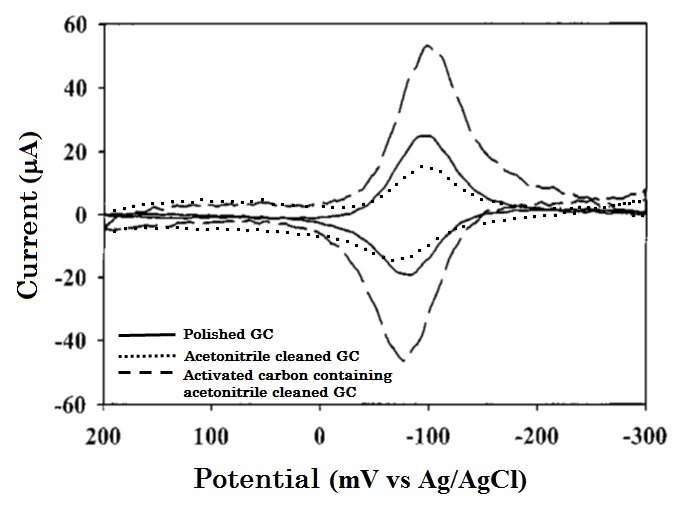
Fig. 7-2 Comparison of differed cleaning treatment GC electrodes for AQDS adsorption redox reactions..
The relevant paper regarding the interferences due to impurities in organic solvents and the validity of activated carbon as a countermeasure is shown in below (Ref. 7-1).
References:
7-1) S.Ranganathan, T.C.Kuo and McCreery, ibid., 71, 3574, (1999).
This is a basic content about the types of working electrodes used for electrochemical measurement, their uses, and selection methods, for beginners in electrochemical measurement.
The topics are listed below:
- Part 1: General Remark
- Part 2: Polarizable electrode and nonpolarizable electrode
- Part 3: Metal electrodes such as gold and platinum
- Part 4: Usually used working electrodes & features
- Part 5: Structure of GC & Electrochemical features of basal/edge plane
- Part 6: Glassy carbon electrode surface state characteristics
- Part 7: Glassy carbon electrode surface impurity adsorption and countermeasures
- Part 8: Adsorption of catechol and derivatives on glassy carbon electrode and electron transfer rate
7. Glassy carbon electrode surface impurity adsorption and countermeasures
Laboratory Of Research & Development, BAS Inc.
Professor Noriyuki Watanabe
Professor Noriyuki Watanabe
An example for oxidation detection of 1 mM ascorbic acid in 0.1 M sulfuric acid by cyclic voltammetry (0.1 V/s) is shown in Fig. 7-1. Although the electron transfer reaction of ascorbic acid is fast, it is a typical example of EC reaction type and is chemically irreversible.
The solid line is for polished GC electrode, the dotted line is for isopropanol cleaned GC electrode, and the dashed line is for isopropanol containing activated carbon cleaned GC electrode, respectively in Fig. 7-1.

Fig. 7-1 Comparison of cyclic voltammograms of ascorbic acid measured by different cleaning treatment GC electrodes.
The oxidation peak potential of ascorbic acid at isopropanol cleaned GC electrode (Fig. 7-1, dotted line) shifts 200 mV to the positive direction comparing with the polished GC electrode (Fig. 7-1, solid line), indicating its electron transfer is slower than the polished GC electrode.
However, the oxidation peak potential of ascorbic acid using activated carbon depredated isopropanol solvent cleaned GC electrode (Fig. 7-1, dashed line) which shifts to the negative direction comparing with polished GC electrode (Fig. 7-1, solid line), indicating that electron transfer becomes faster than the polished electrode, because impurities adsorbed at the GC electrode surface are removed by adsorption to the activated carbon.
Cyclic voltammetry measurement for 40 µM AQDS in 0.1 M HClO4 using polished (solid line), acetonitrile cleaned (dotted line) and acetonitrile containing activated carbon cleaned (dashed line) GC electrodes are compared and shown in Fig. 7-2.
The CV profile exhibits a typical adsorption peak shape which has less attenuation from diffusion, and the small redox peak potential difference. In this case as well as in the previous example, comparing with the polished GC electrode, the peak area of just solvent cleaned GC electrode is decreased. Because the adsorption sites have already been occupied by the impurities in organic solvent, that AQDS can not be adsorbed on GC. Whereas, the peak area is doubled for the GC electrode which cleaned by acetonitrile containing activated carbon (Fig. 7-2, dashed line).

Fig. 7-2 Comparison of differed cleaning treatment GC electrodes for AQDS adsorption redox reactions..
The relevant paper regarding the interferences due to impurities in organic solvents and the validity of activated carbon as a countermeasure is shown in below (Ref. 7-1).
References:
7-1) S.Ranganathan, T.C.Kuo and McCreery, ibid., 71, 3574, (1999).

