Part 7: Electrochemical Impedance Spectroscopy (EIS)
This is a basic introduction to the electrochemical measurement method electrochemical impedance spectroscopy (EIS).
The topics are listed below:
- EIS I: Basis for analysis of EIS results using equivalent circuits
- EIS II: Frequency variation and EIS measurements
- EIS III: Nyquist plot of circuit elements
- EIS IV: EIS IV: Warburg Impedance
- EIS V: Constant Phase Element Nyquist Plot
- EIS VI: Consider a system consisting of three elementary processes
- EIS VII: Nernst diffusion
- EIS VIII: Finite diffusion
- EIS IX: Dye-sensitized solar cell (DSSC) EIS - 1
- EIS X: Dye-sensitized solar cell (DSSC) EIS - 2
- EIS XI: Summary
EIS X: Dye-sensitized solar cell (DSSC) EIS - 2
Professor Noriyuki Watanabe
Electrochemical measurements are basically a three electrodes method, but due to the structural limitations of the object of study, the two electrodes method, which omits the reference electrode, has to be used in many cases. This is the case, for example, with dye sensitized solar cells (DSSC), fuel cells and lithium batteries. The two electrodes method of EIS measurement reflects the combined information of the working electrode and the counter electrode, and cannot distinguish between the measurement of the working electrode and the counter electrode. It is difficult to get information about the working electrode or counter electrode separately in this measurement method. In DSSC, a nanoporous titanium oxide membrane is used as the anode and a Pt electrode is used as the cathode. In addition to the EIS information reflecting the performance of each electrode, contributions such as information on the diffusion of ions in the solution components are added. In an actual DSSC, it is almost impossible to insert a reference electrode in the space between the anode and cathode, so it is difficult to measure the impedance of the anode alone, separated from the cathode.
In this article, an example of a DSSC measurement cell that manages to incorporate a reference electrode is presented 1). Due to the addition of a reference electrode, its structure is slightly different from the actual DSSC, but it is very useful for distinguishing the respective contributions of the cathode and the anode. Fig. 20-1 shows a schematic diagram of the measurement cell. A reference electrode (0.2 mm diameter platinum wire covered with black platinum) is inserted between the anode and the cathode (spaced 1.5 mm apart).
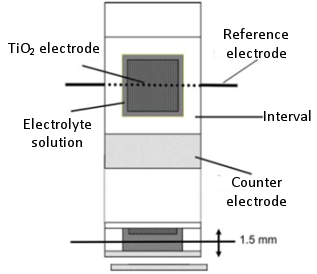
Fig. 20-1 Schematic diagram of three electrodes DSSC.
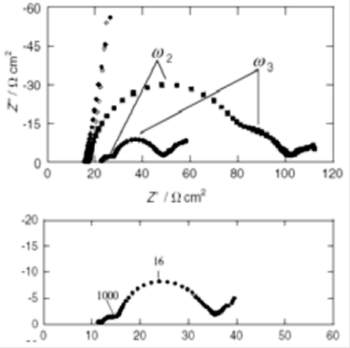
Fig. 20-2 Comparison of total impedance (top) and anode impedance (bottom).
Fig. 20-2 shows the Nyquist plots obtained using the measurement cell. The above figure shows the total impedance of four different cells with different amounts of Pt at the cathode (counter electrode). The total impedance is measured using only the anode and cathode, without a reference electrode, because the arcs of the two lowest Pt contents are too large to be included in the figure (the cathode is a transparent electrode with a different amount of Pt loaded by thermal decomposition). The higher the Pt content, the smaller the arc at high frequencies (indicated as ω2 in the diagram). It is clear that the arc in the high frequency region originates from the cathode. On the other hand, the arc in the middle frequency range (ω3) does not depend on the amount of platinum, so it depends on something other than the cathode.
The lower figure in Fig. 20-2 is a three electrodes measurement using TiO2 as a working electrode, and should not be affected by the counter electrode. The apex frequencies of the arcs are not exactly the same. It may vary slightly. This is, because the frequencies are determined by multiplying the resistive and capacitive components, they are affected independently when conditions are changed. In addition, various studies have been carried out by varying the solution composition, which is an interesting example to understand how these effects are reflected as impedance. For cells that use a high performance counter electrode (because the impedance of the counter electrode will be small), a two electrodes impedance measurement method is often acceptable, but it is still worthwhile to consider a three electrodes cell.
Reference:
20-1) K. Eguchi et al., J. Electroanal. Chem., 588, 59 (2006).

